Folate-mediated and doxorubicin-conjugated poly(ε-caprolactone)-g-chondroitin sulfate copolymers for enhanced intracellular drug delivery†
Yu-Sheng Liua,
Hsuan-Ying Chena,
Jay-An Yeha and
Li-Fang Wang*abc
aDepartment of Medicinal and Applied Chemistry, Kaohsiung Medical University, School of Life Science, 100, Shih-Chuan 1st Rd, Kaohsiung 807, Taiwan. E-mail: lfwang@kmu.edu.tw; Fax: +886-7-3125339; Tel: +886-7-3121101-2217
bDepartment of Biotechnology, Kaohsiung Medical University, Kaohsiung 807, Taiwan
cInstitute of Medical Science and Technology, National Sun Yat-Sen University, Kaohsiung 804, Taiwan
First published on 4th November 2014
Abstract
The aim of this study was to conjugate an anticancer drug, doxorubicin (DOX) and a folate targeting moiety, folic acid (FA), to self-assembled polycaprolactone (PCL)-graft-chondroitin sulfate (CS) copolymers for enhanced chemotherapy. The PCL-graft-CS copolymer was abbreviated as CP. DOX was conjugated to CP using a bifunctional polyethylene glycol as a spacer (CP-DOX). FA was conjugated to the CP-DOX to yield FA-CP-DOX that could enhance the cellular uptake in folate receptor (FR)-overexpressing cancer cells. The CP-DOX and FA-CP-DOX copolymers were confirmed using 1H-nuclear magnetic resonance (1H-NMR) and Fourier transform infrared (FTIR) spectrophotometers. CP-DOX was spherical and FA-CP-DOX was worm-like. The copolymers without DOX were non-cytotoxic against U87 cells. The IC50 value (an inhibitory concentration of 50% cell growth) of FA-CP-DOX was comparable to that of free DOX but much lower than that of CP-DOX against U87 cells 24, 48 and 72 h post incubation. Because of recognition of the FR, the magnificent cellular uptake of FA-CP-DOX into U87 cells was observed using flow cytometry and confocal laser scanning microscopy.
Introduction
The most considerable challenges facing effective cancer therapy are the solubility and systemic toxicity of anticancer agents, the lack of tumor localization and an even distribution throughout the whole body including tumor tissues.1,2 In addition, anticancer drugs' short half-lives in blood circulation and their undesirable pharmacokinetic behavior are among other drawbacks which are present in the way of cancer chemotherapy.3 Conjugation of low molecular weight (MW) drugs to macromolecular carriers is therefore considered a promising approach for improving the efficacy of cytotoxic drugs on tumor cells along with fewer side effects on normal tissues.3–5 A good example of a rational drug-conjugate design is the history of CRLX101,6 which was developed by covalently conjugating camptothecin (CPT) to a linear, cyclodextrin-polyethylene glycol (CD-PEG) copolymer that self-assembles into nanoparticles.The macromolecular conjugated prodrugs have several advantages over their low MW precursors.7 The main advantages are: (1) an increase in the water solubility of low soluble or insoluble anticancer drugs and enhanced biodistribution and therapeutic efficacy; (2) an accumulation of anticancer drugs in tumor tissues through the enhanced permeation and retention (EPR) effect and a reduction in systemic side effects; (3) an improvement in drug pharmacokinetics and a more prolonged plasma half-life; (4) the potential of developing a multifunctional drug delivery system, consisting of several active therapeutic or imaging molecules; (5) protection of anticancer drugs against deactivation during blood circulation, transport to targeted organs or tissues and intracellular trafficking. Polysaccharide-based macromolecular prodrugs have gained increasing attention, such as hyaluronic acid (HA),8–10 dextran,11 chitosan,12 and heparin,13,14 due to their cost effectiveness, abundance in nature, remarkable physicochemical, and biological characteristics and simplicity of chemical reactions required for specific modifications.
Most of the polysaccharides contain reactive functional groups, such as amino, hydroxyl and carboxylic groups, which may readily be utilized as an active site for drug conjugation either directly or via linkers. Chondroitin sulfate (CS) is a natural polysaccharide widely distributed in the human organism. It binds endogenous proteins with different functional properties such as growth factors, adhesion molecules or enzymes, which regulate the human immune system.15 CS has a similar chemical structure to HA, which can recognize CD44 receptors overexpressing in many solid tumor cells.16,17 Besides, CS has many promising properties including biocompatibility, biodegradability, an anti-inflammatory agent.18
Our previous study successfully introduced methacrylate groups to CS (CSMA) that could be reacted with poly(caprolactone) (PCL) end-capped double bonds to yield a graft copolymer. Various amounts of PCL have been grafted onto CSMA with different degrees of methacrylation and the chemicophysical properties and morphologies of PCL-graft-CS copolymers (CP) were thoroughly characterized.19,20 It appears worthwhile to use PCL as a core and CS as a shell because CS is a highly water-soluble polymer and PCL is a highly hydrophobic polymer, leading to a lower critical micelle concentration that benefits the stabilization of micelles circulation in the bloodstream. Two anticancer drugs have been loaded in the core of the self-assembled CP micelles for delivering doxorubicin (DOX)21 or CPT22 to suppress the growth of non-small cell lung tumors. The CP micelles are expected to be biodegradable due to the susceptibility of the aliphatic ester of PCL and the sugar linkages of CS to hydrolysis.
In this contribution, an optimized composition of PCL on CP was synthesized via a free radical reaction. Because CP bears both of carboxylic and hydroxyl groups to be utilized to conjugate with anticancer drugs, thus, an anticancer agent, DOX, was chemically linked to CP to produce CP-DOX. Further, a cancer mediated ligand, folic acid (FA), was grafted onto CP-DOX to improve cellular uptake into tumor cells, which overexpress folate receptors (FR). In future, same or different hydrophobic anticancer agents can be loaded into the core of the self-assembled CP micelles to test the synergetic effect in chemotherapy.
Experimental
Materials
Sodium chondroitin sulfate was purchased from Tohoku Miyagi Pharmaceutical Co., Ltd. (Tokyo, Japan). Methacrylic anhydride and acryloyl chloride were purchased from Lancaster (Lancashire, UK) and used as received. Pyrene, ε-caprolactone, 2,2′-azobisisobutyronitrile (AIBN), and 1,1′-carbonyldiimidazole (CDI) were purchased from Acros (Geel, NJ). Bi-functional poly(ethylene glycol) (NH2-PEG-NH2, MW = 3350 g mol−1) and (NH2-PEG-COOH, MW = 3500 g mol−1) were purchased from Sigma chemical company (St. Louis, MO) and JenKem technology (Beijing, China), respectively. Doxorubicin (DOX), dimethyl sulfoxide-d (DMSO-d6), chloroform-d (CDCl3), and deuterium oxide (D2O) were purchased from Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was purchased from Biological Industries (Beit Haemek, Israel). Potassium dihydrogen phosphate, disodium hydrogen phosphate, glycine, boric acid, and hydrochloric acid were purchased from Fluka (Buchs, Switzerland) and used for buffer preparation. Roswell park memorial institute medium (RPMI 1640) and trypsin-EDTA were obtained from Invitrogen (Carlsbad, CA). All other unstated chemicals were purchased from Sigma chemical company and used without further purification.Preparing methacrylated chondroitin sulfate (CSMA), poly(ε-caprolactone) end-capped with acrylated groups (MeO-PCL-Ac) and poly(ε-caprolactone)-g-chondroitin sulfate (CP)
CSMA and MeO-PCL-Ac were synthesized according to our previous report.21 The degree of methacrylation on CS was controlled at 70%. CP copolymers were synthesized using a free radical reaction as previously described.21 Briefly, AIBN (1 wt%) in DMSO relative to the total weight of MeO-PCL-Ac and CSMA was added to the solution, where CSMA (100 mg) with 1 mL of double deionized (DD) water and MeO-PCL-Ac (100 mg) with 100 mL of dimethylsulfoxide (DMSO) were stirred in a two-neck round-bottom flask under an argon atmosphere at 60 °C. Following 8 hours reaction at 60 °C, the reaction solution was cooled to room temperature and purified by dialysis against DD water using MW cut-off 6000–8000 membrane (Spectrum Labs, Rancho Dominguez, CA) for 2 days. The dialyzed solution was removed from the dialysis tube to a serum bottle, followed by freeze-drying. The residue of MeO-PCL-Ac was removed by washing three times with ethyl acetate. Next, the precipitate was dissolved in DD water and centrifuged for 5 minutes at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The supernatant was removed and lyophilized to yield the final CP (yield: ∼53%).
000 rpm. The supernatant was removed and lyophilized to yield the final CP (yield: ∼53%).
Preparing folic acid-poly(ethylene glycol) (FA-PEG) and doxorubicin-poly(ethylene glycol) (DOX-PEG)
Preparing folic acid-poly(ethylene glycol)-CP (FA-PEG-CP), CP-poly(ethylene glycol)-doxorubicin (CP-DOX) and folic acid-poly(ethylene glycol)-CP-poly(ethylene glycol)-doxorubicin (FA-CP-DOX)
Characterization
FTIR spectra were acquired using a Perkin-Elmer-2000 spectrometer. Dried samples were mixed with potassium bromide (KBr) powder and pressed into pellets. Sixty-four scans were signal-averaged in the range of 4000–400 cm−1 at a resolution of 4 cm−1. The chemical structures of samples were determined using proton nuclear magnetic resonance (1H-NMR) spectroscopy (Varian Mercury plus-200 spectrometer, Varian; Palo Alto, CA), where CSMA, FA-PEG, FA-PEG-CP, DOX-PEG, CP-DOX, and FA-CP-DOX were dissolved in D2O, CP in DMSO-d6, and MeO-PCL-Ac in CDCl3, respectively.Preparing and characterizing micelles
Micelles were prepared by a dialysis method. Ten mg of copolymers were dissolved in 5 mL of DMSO containing 4 μL of trifluoroacetic acid at 60 °C. The solution was dialyzed against DD water and freeze-dried to yield a micellar product. A critical micelle concentration (CMC) value was obtained using pyrene as a hydrophobic fluorescence probe. Pyrene at 3.0 × 10−5 M in acetone was prepared as a stock solution. Sixty μL of the stock solution were added into each vial and acetone was removed by hair-drier. A series of micelles in DD water (1.91 × 10−6 to 1.0 mg mL−1) were added into the vial containing pyrene and stored in darkness overnight. Pyrene excitation spectra were recorded using an emission wavelength at 390 nm. The emission and excitation slit widths were set at 2.5 and 2.5 nm, respectively. The CMC value was determined from the ratios of pyrene intensities at 339 and 334 nm and calculated from the intersection of two tangent plots of I339/I334 vs. the log concentrations of micelles.19Micelles were suspended in DD water at a concentration of 0.5 mg mL−1 and hydrodynamic diameters were measured three times using dynamic light scattering (DLS, Zetasizer Nano ZS, Malvern; Worcestershire, UK). The morphologies of micelles were observed by transmission electron microscopy (JEM-2000 EXII; JEOL, Tokyo, Japan). A carbon coated 200 mesh copper specimen grid (Agar Scientific Ltd. Essex, UK) was glow-discharged for 1.5 minutes. Each micelle solution (0.5 mg mL−1) was dropped on a copper grid and allowed to dry at room temperature for 3 days.
Drug release
The in vitro release of DOX from CP-DOX was determined in 0.1 M PBS buffers of pH 5.6, 6.4, and 7.4 at 37 °C. One mg of CP-DOX was suspended in 1 mL of the above buffers. Each Eppendorf was kept in a shaker at 37 °C and 150 rpm. At predetermined time intervals, four Eppendorfs were removed from the shaker and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C for 5 min. The supernatants were collected to estimate the amount of DOX release, which was correlated to a standard calibration curve of DOX in the same buffer using a UV-Vis spectrophotometer, as aforementioned.
000 rpm and 4 °C for 5 min. The supernatants were collected to estimate the amount of DOX release, which was correlated to a standard calibration curve of DOX in the same buffer using a UV-Vis spectrophotometer, as aforementioned.
Cell experiments
U87 cells (a human glioblastoma cell line) were cultured in RPMI 1640 supplemented with 10% FBS and 1% penicillin-streptomycin at 37 °C under humidified 5% CO2. The medium was replenished every three days. The cells were sub-cultured after they had reached confluence. Live cells of U87 were counted using a trypan-blue dye exclusion method23 after the cells had been incubated with CP, DOX, FA-CP-DOX, and CP-DOX, respectively.The cells were seeded in 12-well culture plates at a density of 1 × 105 cells per well in RPMI 1640 containing 10% FBS for 24 hours. The culture medium was removed and replaced with 1 mL medium containing various concentrations of test samples. Following 24, 48 and 72 hours of incubation, the cells were washed three times with PBS and trypsinized. The cells were collected and resuspended with 1 mL of PBS in an Eppendorf. The 10 μL PBS solution containing the cells was mixed with 10 μL trypan blue and live cells were observed using a microscope (Nikon Eclipse TS100; Tokyo, Japan).
Flow cytometry
The cellular uptake of free DOX, CP-DOX and FA-CP-DOX was studied using a flow cytometer. U87 cells were seeded at a density of 1 × 105 cells per well in 6-well plates in RPMI 1640 supplemented with 10% FBS and incubated for 24 hours. Next, the culture medium was removed and replaced with 2 mL RPMI 1640 containing test samples at a DOX concentration of 2 μg mL−1. The cells were further incubated at 37 °C for 2, 6 and 12 hours, washed three times with PBS, collected and analyzed using an EPICS XL flow cytometer (Beckman Coulter, Fullerton, CA).The competitive inhibition of folate receptors was assayed by flow cytometric analysis. U87 cells were incubated in folic acid-deficient RPMI 1640 supplemented with 10% FBS and 1 mL of free FA (2 mg mL−1) for 24 hours. The medium was removed before 2 mL of folic acid-deficient RPMI 1640 containing test samples at a DOX concentration of 2 μg mL−1 were added and incubated for 2 hours. Otherwise the cells were treated as same procedures as described above.
Confocal laser scanning microscopy (CLSM)
U87 cells were seeded at a density of 1 × 105 cells per well in 12-well plates containing one glass coverslip per well, in RPMI 1640 supplemented with 10% FBS for 24 hours. The 1 mL of RPMI 1640 (without 10% FBS) containing free DOX, FA-CP-DOX and CP-DOX at a DOX concentration of 2 μg mL−1 was added into each well and incubated at 37 °C for 2 hours. The coverslips containing the cells were removed and washed gently with 2 mL of 0.1 M PBS (twice), treated with 5 μg mL−1 Hoechst 33342 to stain cell nuclei for 30 minutes and 100 nM Lysotracker Green (DND-26, Invitrogene; Carlsbad, CA) to stain endolysosomes for 1 hour. Next, the cells were washed three times with 0.1 M PBS and fixed with 3.7% paraformaldehyde for 30 minutes. The cells on the coverslips were washed three times with 0.1 M PBS and mounted with fluorescent mounting medium on glass slides for CLSM (Fv 1000; Olympus, Tokyo, Japan) observation.Statistical methods
The results were reported as mean ± standard deviation. Statistical significance was determined by two-tailed Student t-test. *P < 0.05 was considered to be significant.Results and discussion
Synthesis of CP-DOX and FA-CP-DOX
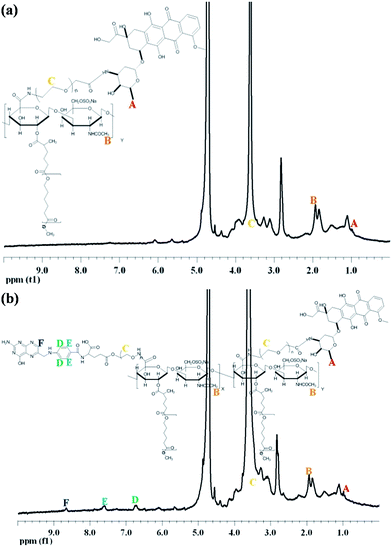
Amphiphilic copolymers combining extremely different hydrophilic and hydrophobic characteristics between CS and PCL have been successfully synthesized.19,21 Instead of using a PCL molecular weight of 2200 g mol−1 in previous studies, we used 3500 g mol−1 to increase the hydrophobic core area to accommodate more hydrophobic drugs for future studies. The weight ratio of the modified CS and PCL at 1/1 in feed was adapted to produce PCL-graft-CS (CP). The FT-IR spectrum of CP shows the characteristic –OH stretching signal in the 3200–3600 cm−1 region and the maximum peak intensity of –CONH at 1646 cm−1 mainly attributed to CS. The carbonyl –COO absorbance at 1726 cm−1 was assigned to PCL. The PCL composition of CP was characterized by 1H-NMR, that is 8.1 mol%. When the hydrophilic content is larger than the hydrophobic content, a micellar architecture is commonly observed.24To link FA to CP, PEG was utilized as a spacer. The PEG diamine and FA at a molar ratio of 1 was set to produce FA-PEG using CDI as a coupling agent, slightly modified from the method reported in the literature.25 The 1H-NMR spectrum of FA-PEG shows the peaks at 6.6, 7.7 and 8.5 ppm because of the FA moiety and 3.8 ppm because of the methylene units of PEG (ESI Fig. S1†). The peak intensity ratio of FA and PEG was measured and used to estimate the degree of FA linked to PEG, i.e. ∼83.3%. In parallel, a standard calibration curve of FA was built using a UV-Vis spectrophotometer at 280 nm. The percentage of FA in FA-PEG calculated against the FA calibration curve (R2 = 0.9987) is ∼96.7%, assuming that the absorption coefficient of FA-PEG was the same as FA.26 Both NMR and UV-Vis results implied that approximately one end of PEG was modified with FA.
FA-PEG-grafted CP (FA-PEG-CP) was synthesized using the amino groups on the end of FA-PEG to react with the carboxylic groups of CP using EDAC as a conjugation agent. The graft percentage of FA-PEG onto CP was tested with various feeding weight ratios of FA-PEG and CP as shown in Table 1. The FA-PEG content of FA-PEG-CP was determined against the calibration curve done with FA-PEG using the UV-Vis spectrophotometer at 280 nm (R2 = 0.9998). The result demonstrated that the FA-PEG content of FA-PEG-CP increases linearly through increasing the FA-PEG feeding amount (R2 = 0.9918, ESI Fig. S2†).
FA-PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP (weight ratio) CP (weight ratio) |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
|---|---|---|---|---|---|
| Absorbancea | 0.462 | 0.493 | 0.505 | 0.528 | 0.570 |
| Concentration (mg mL−1) | 0.058 | 0.062 | 0.064 | 0.066 | 0.072 |
| μM | 15.30 ± 0.15 | 16.48 ± 0.17 | 16.90 ± 0.31 | 17.64 ± 0.33 | 19.06 ± 0.18 |
The 1H-NMR and FTIR spectra of FA-PEG-CP synthesized at the weight ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 of FA-PEG and CP were shown in Fig. 1. The peaks appearing at 6.6, 7.7, and 8.5 ppm are attributable to FA, 3.8 ppm to PEG, and 1.9 ppm to the acetamide group of CS (Fig. 1a). Since the NMR spectrum was done in D2O, the characteristic peaks of PCL are unclear due to the solubility issue. The FT-IR spectrum of FA-PEG-CP appears to be a combination from the spectra of CP and FA-PEG (Fig. 1b). The –OH stretching in the 3100–3600 cm−1 region and the maximum signal of carbonyl –COO absorbance at 1730 cm−1 were attributed to CP and the C–O–C stretching at 1103 cm−1 was attributed to FA-PEG. The FA-PEG concentration of FA-PEG-CP obtained from the UV-Vis measurement is 10.87 μM. This weight ratio of 30
30 of FA-PEG and CP were shown in Fig. 1. The peaks appearing at 6.6, 7.7, and 8.5 ppm are attributable to FA, 3.8 ppm to PEG, and 1.9 ppm to the acetamide group of CS (Fig. 1a). Since the NMR spectrum was done in D2O, the characteristic peaks of PCL are unclear due to the solubility issue. The FT-IR spectrum of FA-PEG-CP appears to be a combination from the spectra of CP and FA-PEG (Fig. 1b). The –OH stretching in the 3100–3600 cm−1 region and the maximum signal of carbonyl –COO absorbance at 1730 cm−1 were attributed to CP and the C–O–C stretching at 1103 cm−1 was attributed to FA-PEG. The FA-PEG concentration of FA-PEG-CP obtained from the UV-Vis measurement is 10.87 μM. This weight ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 of FA-PEG and CP was adapted to link DOX molecules in the later study.
30 of FA-PEG and CP was adapted to link DOX molecules in the later study.
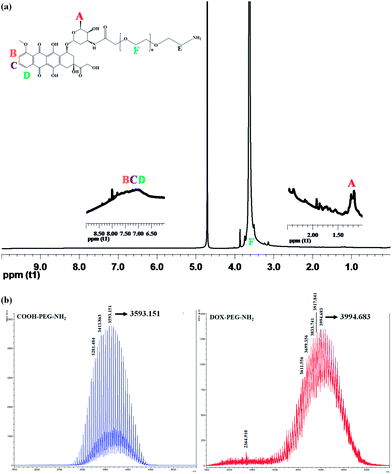
Doxorubicin-poly(ethylene glycol) (DOX-PEG) was synthesized using the method modified from the literature.27 In the 1H-NMR spectrum of DOX-PEG (Fig. 2a), the presence of DOX was confirmed by the appearance of signals at 6.5–8.5 ppm (Peaks B, C and D), attributable to the aromatic ring, and 1.18 ppm (Peak A), attributable to the methyl groups of DOX. The MALDI-MS spectrum exhibits the maximum peak of COOH-PEG-NH2 at 3593.1 (Fig. 2b, left panel), close to the reported molecular weight of 3500 g mol−1 from the supplier (JenKem technology). The molecular weight of DOX is 543.5 g mol−1. After the conjugation reaction occurring between COOH-PEG-NH2 and DOX, one mole of water was given off. Thus, the theoretical molecular weight of DOX-PEG is 4018.6 g mol−1. The MALDI-MS spectrum of DOX-PEG (Fig. 2b, right panel) shows the maximum peak at 3994.6 m/z, close to the theoretical value. A standard calibration curve of DOX was created using the UV-Vis spectrophotometer at 485 nm. The percentage of DOX in DOX-PEG calculated against the DOX calibration curve (R2 = 0.9998) is ∼68.5%.
The amino groups on the end of DOX-PEG were utilized to react with the carboxylic groups of CP to yield CP-DOX. Similarly, the amino groups of DOX-PEG and FA-PEG were simultaneously reacted with CP to yield FA-CP-DOX. Their 1H-NMR spectra were shown in Fig. 3. In the 1H-NMR of CP-DOX (Fig. 3a), Peak A is a characteristic signal of the methyl group of DOX, Peak B is attributed to the acetamide of CS and Peak C is the ethylene linkage of PEG. In addition to the characteristic peaks, attributable to CS, DOX and PEG, the 1H-NMR spectrum of FA-CP-DOX shows the FA peaks of para-aminobenzoic acid (Peaks D and E) and aromatic pteridine ring (Peak F) at δ 6.80, 7.60 and 8.60 ppm, respectively. The mol% of DOX measured against the DOX calibration curve are 8.34% for CP-DOX and 8.12% for FA-CP-DOX, respectively.
Micellar properties and drug release
Hydrophobic amphiphilic copolymers could be assembled through a simple dialysis method to form micelles in an aqueous solution because of easy segregation between hydrophobic and hydrophilic segments.28 The CMC value of CP is 3.08 μg mL−1 determined using pyrene as a fluorescent probe (Table 2). Modification of CP with FA-PEG dramatically increases a CMC value. The CMC value of FA-PEG-CP goes hand-in-hand with an increase in FA-PEG feeding amount. For example, the CMC value of FA-PEG-CP is 8.13 μg mL−1 with a weight feed ratio of FA-PEG and CP at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and increases to 13.2 μg mL−1 at 50
30 and increases to 13.2 μg mL−1 at 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (Table 2 and ESI Fig. S3†). The introduction of FA-PEG to CP increases the solubility of CP as well (ESI Fig. S4†). At a concentration of 1 mg mL−1 in DD water, CSMA is completely soluble; CP is turbid and FA-PEG-CP at a feed weight ratio of 30
30 (Table 2 and ESI Fig. S3†). The introduction of FA-PEG to CP increases the solubility of CP as well (ESI Fig. S4†). At a concentration of 1 mg mL−1 in DD water, CSMA is completely soluble; CP is turbid and FA-PEG-CP at a feed weight ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 becomes transparent.
30 becomes transparent.
| Dha (nm) | PDIa | Zeta (mV) | CMC (mg mL−1) | |
|---|---|---|---|---|
a Micelles sizes and polydispersity index (PDI) were measured at a concentration of 0.1 mg mL−1 in DD water by DLS. CMC: critical micellar concentration; ND: not determined.b CP-DOX and FA-CP-DOX were prepared at the weight ratio of FA-PEG and CP at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. 30. |
||||
| CP | 231.2 ± 30.5 | 0.404 ± 0.02 | −42.2 ± 2.8 | 3.08 × 10−3 |
FA-PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CP (5 CP (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
236.9 ± 24.5 | 0.352 ± 0.03 | −21.5 ± 2.6 | 8.13 × 10−3 |
(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
215.1 ± 17.2 | 0.329 ± 0.03 | −22.6 ± 3.1 | 8.33 × 10−3 |
(15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
214.6 ± 15.1 | 0.298 ± 0.02 | −24.2 ± 5.7 | 8.61 × 10−3 |
(30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
222.7 ± 9.5 | 0.215 ± 0.03 | −22.7 ± 5.1 | 9.67 × 10−3 |
(50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
208.2 ± 8.1 | 0.257 ± 0.01 | −20.5 ± 2.1 | 1.32 × 10−2 |
| CP-DOXb | 253.3 ± 21.2 | 0.372 ± 0.08 | −19.5 ± 4.2 | ND |
| FA-CP-DOXb | 241.2 ± 24.5 | 0.318 ± 0.09 | −17.6 ± 1.2 | ND |
The hydrodynamic diameter of CP is 231 nm and slightly deceases as FA-PEG is grafted onto it. For example, the hydrodynamic diameter of FA-PEG-CP at a weight ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 is ∼208 nm. Incorporation of DOX-PEG to CP increases the hydrodynamic diameter to 253 nm for CP-DOX and 241 nm for FA-CP-DOX (Table 2 and ESI Fig. S5†). The zeta potential of CP is −42 mV and increases to between −20 and −25 mV depending on the amount of FA-PEG grafted onto CP (Table 2). A further graft of DOX-PEG to FA-PEG-CP (FA-CP-DOX) changed the zeta potential to a less negative value because some carboxylic groups of CP had been utilized to react with DOX-PEG, leading to reduced negative charges on the exterior surface of CP.
30 is ∼208 nm. Incorporation of DOX-PEG to CP increases the hydrodynamic diameter to 253 nm for CP-DOX and 241 nm for FA-CP-DOX (Table 2 and ESI Fig. S5†). The zeta potential of CP is −42 mV and increases to between −20 and −25 mV depending on the amount of FA-PEG grafted onto CP (Table 2). A further graft of DOX-PEG to FA-PEG-CP (FA-CP-DOX) changed the zeta potential to a less negative value because some carboxylic groups of CP had been utilized to react with DOX-PEG, leading to reduced negative charges on the exterior surface of CP.
The morphology of the self-assembled CP was examined by TEM as a spheroid (Fig. 4a–c). Apparently, FA-PEG-CP is worm-like using a weight ratio of FA-PEG and CP at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (Fig. 4d–f). After grafting DOX onto CP or FA-PEG-CP, CP-DOX still shows a spherical image (Fig. 4g–i) and FA-CP-DOX is worm-like, a similar appearance to FA-PEG-CP (Fig. 4j–l). The average particle sizes estimated from TEM images are 90.9 ± 7.6, 82.2 ± 9.6, 92.5 ± 3.2, and 87.6 ± 8.2 nm, for CP, FA-PEG-CP, CP-DOX, and FA-CP-DOX, respectively. Since the TEM samples were done in a dehydrated state and those of DLS in a solution, the larger particle diameters observed in DLS could be attributed to the swelling behavior of the shell compartment.29
30 (Fig. 4d–f). After grafting DOX onto CP or FA-PEG-CP, CP-DOX still shows a spherical image (Fig. 4g–i) and FA-CP-DOX is worm-like, a similar appearance to FA-PEG-CP (Fig. 4j–l). The average particle sizes estimated from TEM images are 90.9 ± 7.6, 82.2 ± 9.6, 92.5 ± 3.2, and 87.6 ± 8.2 nm, for CP, FA-PEG-CP, CP-DOX, and FA-CP-DOX, respectively. Since the TEM samples were done in a dehydrated state and those of DLS in a solution, the larger particle diameters observed in DLS could be attributed to the swelling behavior of the shell compartment.29
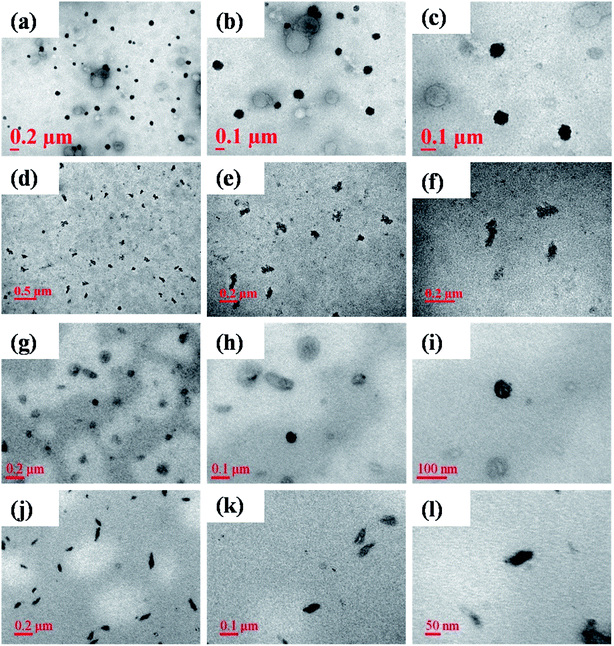 | ||
| Fig. 4 TEM images of (a)–(c), CP; (d)–(f), FA-PEG-CP; (g)–(i), CP-DOX and (j)–(l), FA-CP-DOX with different magnifications. | ||
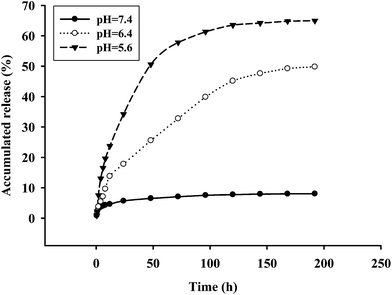
In vitro DOX release from CP-DOX was carried out in 0.1 M PBS and pH of 5.6, 6.4 and 7.4 at 37 °C. As seen in Fig. 5, about 8% of DOX was released at pH 7.4, 50% at pH 6.4, and 65% at pH 5.6 within 180 hours. CP-DOX was expected to have an effective drug release in the tumor microenvironment since the drug release rate accelerated with the decrease in pH. A similar drug released profile was speculated to FA-CP-DOX because it has the same hydrolysable linkage as CP-DOX. Although tumor pH may vary according to the tumor area, average extracellular tumor pH is between 6.0 and 7.0, whereas in normal tissues and blood, the extracellular pH is around 7.4.30,31 In addition, most of the nanoparticles are taken up by endocytosis into cells32 and this endocytic pathway begins near the physiological pH of 7.4, then it drops to pH 5.5–6.0 in endosomes.33 Thus, the pH-dependent release behavior of CP-DOX will benefit tumor-targeted DOX delivery.
Cytotoxicity and intracellular uptake
Cytotoxic data demonstrated that U87 cells are slightly dose dependent on the CP concentration and show ∼93% cell viability, even at a high concentration of 1000 μg mL−1 (ESI Fig. S6†). To test which DOX formulation is more sensitive to U87 cells, the cells exposed to various concentrations of free DOX, CP-DOX and FA-CP-DOX were studied at three incubation time points of 24, 48 and 72 hours (ESI Fig. S7†). The IC50 values, defined as a concentration inhibiting the 50% cell proliferation, were summarized in Table 3. The IC50 values decrease as the incubation time period increases. The values are 3.59, 0.97, and 0.39 μg mL−1 of U87 cells exposed to free DOX for 24, 48 and 72 hours, respectively. Free DOX shows the highest cytotoxicity against U87 cells and FA-CP-DOX is close to free DOX. Both have a better cell-killing effect than CP-DOX.| Sample | Incubate time | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Free DOX | 3.59 μg mL−1 | 0.97 μg mL−1 | 0.39 μg mL−1 |
| FA-CP-DOX | 4.64 μg mL−1 | 0.99 μg mL−1 | 0.47 μg mL−1 |
| CP-DOX | 8.89 μg mL−1 | 1.87 μg mL−1 | 0.92 μg mL−1 |
The folate receptor on the cell membrane was examined after U87 cells had been treated with folate receptor antibody using flow cytometry (ESI Fig. S8†). The pattern was clearly observed a right shift as compared with the control group, explaining the folate receptor antibody strongly interacts with the cells. The result explained that U87 is a folic acid-expressing cell line. Flow cytometric analysis was utilized to study the cellular uptake of free DOX, CP-DOX and FA-CP-DOX into U87 cells at different time points using an equivalent DOX concentration of 2 μg mL−1. A greater shift of a histogram to the right implies a larger amount of DOX was internalized into the cells. The largest shift was always observed in FA-CP-DOX at 3 test time points of 2, 6 and 12 hours (Fig. 6). Clearly, the cellular uptake of nanoparticles gradually increases with time.
 | ||
| Fig. 6 Flow cytometric histograms of free DOX, FA-CP-DOX and CP-DOX relative to U87 control cells at 2, 6 and 12 h of incubation. The equivalent DOX concentration was 2 μg mL−1. | ||
A further comparison of cellular uptake among free DOX, CP-DOX and FA-CP-DOX was carried out using CLSM following 2 h of incubation. As seen in Fig. 7, both CP-DOX and FA-CP-DOX enter into the cytoplasm and localize at the endolysosomes of U87 cells. In contrast, low fluorescence was observed for the cells incubated with free DOX. The possible explanation for the higher uptake in the formulated DOX was that micellar particles are usually internalized inside cells by endocytosis, while free drugs mainly do this by diffusion.34 Moreover, the superior uptake of FA-CP-DOX compared to free DOX as well as CP-DOX might be because FA-CP-DOX recognizes FR, which facilitates FR-mediated endocytosis. To confirm this internalization route, the cells were pretreated with FA to block the FR-mediated uptake process, prior to incubation with CP-DOX or FA-CP-DOX. A clear decrease in uptake was observed in FA-CP-DOX alone (Fig. 8), indicating that the FR-mediated endocytosis pathway was indeed involved in the internalization of FA-CP-DOX into U87 cells.
 | ||
| Fig. 8 Flow cytometric histograms of FA-CP-DOX and CP-DOX in the presence and absence of folic acid in U87 cells at 2 h of incubation. The equivalent DOX concentration was 2 μg mL−1. | ||
Optimizing the drug release from drug-encapsulated nanoparticles after entering inside cells is also a key factor in designing a successful drug delivery system. In addition to chemically-loaded DOX to CP-DOX and FA-CP-DOX, the hydrophobic core area of CP-DOX and FA-CP-DOX could be utilized to physically load a hydrophobic drug to increase the synergistic effect in chemotherapy in future.
Conclusions
We successfully conjugated CP with FA-PEG and DOX-PEG to produce FA-CP-DOX as macromolecular prodrugs as well as hydrophobic drug carriers. Modification of CP with FA-PEG dramatically increased a CMC value, which increased with an increase in FA-PEG content. The hydrodynamic diameter decreased with an increased FA-PEG content. Conjugation of DOX to CP or FA-PEG-CP increased the hydrodynamic diameters as compared to those without DOX. The zeta potential of CP became less negative as FA-PEG was grafted onto its surface. A graft of DOX to FA-PEG-CP changed the zeta potential to a less negative value. From TEM images, CP and DOX-CP were spherical, but FA-PEG-CP and FA-CP-DOX were worm-like. DOX-CP and FA-CP-DOX were expected to have an effective drug release in the tumor microenvironment because the drug release rate accelerated with the decrease in pH. FA-CP-DOX showed the comparable cytotoxicity to free DOX against U87 cells. In addition, FA-CP-DOX displayed the highest DOX internalization among the three test samples, DOX, CP-DOX and FA-CP-DOX. The internalization pathway of FA-CP-DOX was involved with FR-mediated endocytosis in FR-expressing U87 cells. In this study, a new type of amphiphilic FA-CP-DOX copolymer was successfully prepared and its potential application as a macromolecular prodrug as well as a drug carrier was illustrated.Conflict of interest
The authors declare no conflict of interest.Acknowledgements
We are grateful for the financial support from the Ministry of Science and Technology of Taiwan (NSC100-2320-B-037-003-MY3, NSC101-2325-B037-006, NSC102-2325-B037-005, and NSC103-2325-B037-001). This study is also supported by “Aim for the Top 500 Universities Grant” under the grant number KMU-DT103007 from Kaohsiung Medical University.References
- H. Zhang, J. Wang, W. Mao, J. Huang, X. Wu, Y. Shen and M. Sui, J. Controlled Release, 2013, 166, 147–158 CrossRef CAS PubMed.
- H. Xiao, H. Song, Q. Yang, H. Cai, R. Qi, L. Yan, S. Liu, Y. Zheng, Y. Huang, T. Liu and X. Jing, Biomaterials, 2012, 33, 6507–6519 CrossRef CAS PubMed.
- F. Kratz, Expert Opin. Invest. Drugs, 2007, 16, 855–866 CrossRef CAS PubMed.
- S. Zhu, L. Qian, M. Hong, L. Zhang, Y. Pei and Y. Jiang, Adv. Mater., 2011, 23, 84–89 CrossRef PubMed.
- W. L. Ye, J. B. Du, B. L. Zhang, R. Na, Y. F. Song, Q. B. Mei, M. G. Zhao and S. Y. Zhou, PLoS One, 2014, 9, e97358 Search PubMed.
- C. Young, T. Schluep, J. Hwang and S. Eliasof, Curr. Bioact. Compd., 2011, 7, 8–14 CrossRef CAS PubMed.
- N. Goodarzi, R. Varshochian, G. Kamalinia, F. Atyabi and R. Dinarvand, Carbohydr. Polym., 2013, 92, 1280–1293 CrossRef CAS PubMed.
- N. Goodarzi, M. H. Ghahremani, M. Amini, F. Atyabi, S. N. Ostad, N. Shabani Ravari, N. Nateghian and R. Dinarvand, Chem. Biol. Drug Des., 2014, 83, 741–752 CAS.
- S. Manju and K. Sreenivasan, J. Colloid Interface Sci., 2011, 359, 318–325 CrossRef CAS PubMed.
- S. Cai, S. Thati, T. R. Bagby, H. M. Diab, N. M. Davies, M. S. Cohen and M. L. Forrest, J. Controlled Release, 2010, 146, 212–218 CrossRef CAS PubMed.
- S. Mitra, U. Gaur, P. C. Ghosh and A. N. Maitra, J. Controlled Release, 2001, 74, 317–323 CrossRef CAS.
- F. Q. Hu, L. N. Liu, Y. Z. Du and H. Yuan, Biomaterials, 2009, 30, 6955–6963 CrossRef CAS PubMed.
- I.-K. Park, Y. J. Kim, T. H. Tran, K. M. Huh and Y.-k. Lee, Polymer, 2010, 51, 3387–3393 CrossRef CAS PubMed.
- W. She, N. Li, K. Luo, C. Guo, G. Wang, Y. Geng and Z. Gu, Biomaterials, 2013, 34, 2252–2264 CrossRef CAS PubMed.
- V. R. Pipitone, Drugs Exp. Clin. Res., 1991, 17, 3–7 CAS.
- A. Pathak, P. Kumar, K. Chuttani, S. Jain, A. K. Mishra, S. P. Vyas and K. C. Gupta, ACS Nano, 2009, 3, 1493–1505 CrossRef CAS PubMed.
- T. Kurosaki, T. Kitahara, S. Kawakami, K. Nishida, J. Nakamura, M. Teshima, H. Nakagawa, Y. Kodama, H. To and H. Sasaki, Biomaterials, 2009, 30, 4427–4434 CrossRef CAS PubMed.
- N. Volpi, Curr. Med. Chem.: Anti-Inflammatory Anti-Allergy Agents, 2005, 4, 221–234 CrossRef CAS.
- A. L. Chen, H. C. Ni, L. F. Wang and J. S. Chen, Biomacromolecules, 2008, 9, 2447–2457 CrossRef CAS PubMed.
- L. F. Wang, H. C. Ni and C. C. Lin, J. Biomater. Sci., Polym. Ed., 2011, 23, 1821–1842 Search PubMed.
- Y. J. Lin, Y. S. Liu, H. H. Yeh, T. L. Cheng and L. F. Wang, Int. J. Nanomed., 2012, 7, 4169–4183 CAS.
- Y. S. Liu, C. C. Chiu, H. Y. Chen, S. H. Chen and L. F. Wang, Mol. Pharm., 2014, 11, 1164–1175 CrossRef CAS PubMed.
- R. Khonkarn, S. Mankhetkorn, W. E. Hennink and S. Okonogi, Eur. J. Pharm. Biopharm., 2011, 79, 268–275 CrossRef CAS PubMed.
- K. Letchford and H. Burt, Eur. J. Pharm. Biopharm., 2007, 65, 259–269 CrossRef CAS PubMed.
- G. Xiang, J. Wu, Y. Lu, Z. Liu and R. J. Lee, Int. J. Pharm., 2008, 356, 29–36 CrossRef CAS PubMed.
- H. Zhu, F. Liu, J. Guo, J. Xue, Z. Qian and Y. Gu, Carbohydr. Polym., 2011, 86, 1118–1129 CrossRef CAS PubMed.
- H. S. Yoo and T. G. Park, J. Controlled Release, 2004, 100, 247–256 CrossRef CAS PubMed.
- C. Allen, D. Maysinger and A. Eisenberg, Colloids Surf., B, 1999, 19, 3–27 CrossRef.
- M. Bodnar, J. F. Hartmann and J. Borbely, Biomacromolecules, 2006, 7, 3030–3036 CrossRef CAS PubMed.
- F. Danhier, O. Feron and V. Préat, J. Controlled Release, 2010, 148, 135–146 CrossRef CAS PubMed.
- M. Breunig, S. Bauer and A. Goepferich, Eur. J. Pharm. Biopharm., 2008, 68, 112–128 CrossRef CAS PubMed.
- T. Tanaka, S. Shiramoto, M. Miyashita, Y. Fujishima and Y. Kaneo, Int. J. Pharm., 2004, 277, 39–61 CrossRef CAS PubMed.
- J. S. Park, T. H. Han, K. Y. Lee, S. S. Han, J. J. Hwang, D. H. Moon, S. Y. Kim and Y. W. Cho, J. Controlled Release, 2006, 115, 37–45 CrossRef CAS PubMed.
- N. Nishiyama and K. Kataoka, Adv. Polym. Sci., 2006, 193, 67–101 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra12187b |
| This journal is © The Royal Society of Chemistry 2014 |