Recycling of waste printed circuit boards into ion exchange resin
Abstract
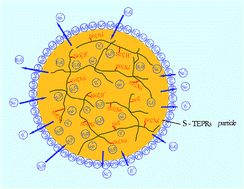
Recycling the nonmetal components of waste printed circuit boards (WPCBs), mainly thermosetting epoxy resins (TEPRs), is quite difficult because they are insoluble and inflexible. We report a new method to convert TEPRs into ion exchange resin by treatment with sulphuric acid and the equilibrium ion exchange capacity (IEC) of the produced sample is 1.63 meq g−1. FTIR indicated that TEPRs were modified by sulphuric acid and X-ray photoelectron spectroscopy (XPS) verified that sulfonic acid group (–SO3−) was introduced. The produced ion exchange resin was stable up to 200 °C by TG-DTG analysis. The maximum adsorption capacities for Cu(II) and Ca(II) were 23.30 and 56.27 mg g−1, respectively. The Langmuir model gave a better fit for adsorption than the Freundlich model. The activation energies (Ea) were 21.18 and 48.53 kJ mol−1 for Cu(II) and Ca(II), respectively, with pseudo-second-order kinetics.

 Please wait while we load your content...
Please wait while we load your content...