Water-soluble non-polymeric electrospun cyclodextrin nanofiber template for the synthesis of metal oxide tubes by atomic layer deposition†
Asli Celebiogluab,
Sesha Vempati*a,
Cagla Ozgit-Akgunab,
Necmi Biyikliab and
Tamer Uyar*ab
aUNAM-National Nanotechnology Research Center, Bilkent University, Ankara, 06800, Turkey. E-mail: svempati01@qub.ac.uk; uyar@unam.bilkent.edu.tr
bInstitute of Materials Science and Nanotechnology, Bilkent University, Ankara, 06800, Turkey
First published on 10th November 2014
Abstract
We report on the suitability of water-soluble non-polymeric electrospun cyclodextrin (CD) nanofiber templates by using atomic layer deposition (ALD) to yield metal oxide tubes. To demonstrate this, water-soluble electrospun CD nanofibers were chosen as template to produce metal oxide tubes where we have tested two examples of ALD coatings, namely, Al2O3 and ZnO. After the ALD coating on the CD nanofibers, the CD core is simply dissolved in water to yield metal oxide tubes. Morphological investigations suggested that Al2O3 is smoother in contrast to ZnO which shows a grainy structure. Structural characterization evidenced amorphous Al2O3 and highly crystalline ZnO. Given the applicability of Al2O3 and ZnO in various contexts the ionic states of Al, Zn and O are also investigated. After the washing step to remove the CD core, Al2O3 developed some hydroxylation, while ZnO hosts various oxygen related functional groups.
Introduction
Nanostructures produced while combining industrially applicable techniques such as electrospinning and atomic layer deposition (ALD) are quite attractive as perused by various researchers for potential applications.1–10 In fact ALD can yield coral,6 core–shell,4,5,11 hollow6,7,9 like complex nanostructures. Although various polymers are the subjects of electrospinning, the compatibility between the polymer and the ALD precursor is vital.2 The foremost condition is the thermal stability of the polymer as ALD generally takes places at slightly elevated temperatures. The other factor is the chemical compatibility, see the ALD of Al2O3 on nylon-6 polymer,2 ALD of ZnO on poly(propylene) fibers with Al2O3 base layer to inhibit the diffusion of diethylzinc (DEZn) into the polymer.1 Despite of these limitations fibers of various polymers are explored by our group3,4,12 and other researchers in ALD.1,2,6,7,9,10 Viz. poly(propylene)/Al2O3/ZnO,1 poly(acrylonitrile)/ZnO,3,10 polysulfone/ZnO,4 nylon 6,6/ZnO,12 nylon-6/ZnO/Al2O3,2 poly(vinyl alcohol) (PVA)/Al2O3,6,7 polyvinyl acetate/ZnO.9 Although electrospun polymeric nanofibers/ALD is established in the literature, the same combination with non-polymeric nanofibers is certainly novel and potential. In the context of electrospinning of non-polymeric systems, cyclodextrin (CD)13–16 are quite interesting apart from others.17,18 Hydrogen-bond-mediated aggregates of CD molecules help the electrospinning process where the aggregation is analogous to the polymeric chain entanglement. These CD aggregates are big enough to mimic the entanglement so that a continuous non-woven nanofibrous mat can be obtained.13–16 Hence in this study we demonstrate the potential compatibility of CD nanofibers in ALD process while exploiting its fascinating properties. For instance, the intrinsic nature of CD helps in sustaining the fiber structure until ∼150 °C, a typical temperature for an ALD process. This is in contrast to water-soluble polymers such as PEO which has melting point of ∼65 °C or PVA with a glass transition temperature less than 100 °C. Hence the present combination is quite potential to produce metal oxide tubes for various applications, especially sensors.9,10 Basically, after the ALD process the core–shell fiber structure is either subjected to calcination9,7 or dissolution6 to remove the core–polymer yielding tube structure. Dissolution of the ‘core’ is quite interesting and energy efficient in contrast to the calcination, while on the other hand the latter strongly manipulates the crystalline property of the shell material. Hence it is always encouraging to increase the applicability of water processible materials by ever increasing environmental and cost related issues (green chemistry). Particularly, CDs are non toxic materials and hence their release into the environment (after the washing step) does not cause any loading. When compared to the polymeric systems, the CD nanofibers have relatively higher and rapid solubility with small molecular size which enables a better removal from core–shell structure. Furthermore, prolonged exposure of metal oxide surfaces to solvents can influence the surface chemical characteristics. We do acknowledge that water-soluble polymers such as PVA6 fiber template has been used to obtain Al2O3 tubes via ALD process however, not many other reports are seen. This study is significant in terms of employing a non-polymeric template as well as demonstrating the compatibility with ALD process to produce metal oxide tubes.In this report we adopted this combination to produce core–shell structured CD–metal oxide fibers and subsequently they were subjected to washing which removes the CD core. Among various nanostructures, tubes have attracted a lot of attention due to their potential applicability. We have used methyl-β-cyclodextrin (MβCD) and hydroxypropyl-β-cyclodextrin (HPβCD) for the electrospinning since they have a very high solubility when compared to native β-CD. We use these electrospun CD nanofibers as templates to obtain Al2O3 and ZnO tubes by ALD coating where the core–shell structure is subjected to a simple dissolution of CD core.
Experimental
Materials
HPβCD (molar substitution ∼0.6) and MβCD (molar substitution 1.6–1.9) were purchased from Wacker Chemie AG, Germany. N,N-dimethylformamide (DMF) was purchased from Riedel, Pestenal. Trimethylaluminum (TMAl) and diethylzinc (DEZn) were procured from Sigma Aldrich and HPLC-grade deionized water were used for the ALD process. All the materials were used without any purification.Fabrication of metal oxide tubes
The process of obtaining metal oxide tubes consists of three steps. Initially CD nanofibers are produced (Step 1, electrospinning), followed by the deposition of metal oxide (Step 2, ALD process). Finally in Step 3, the core–shell nanofibers are subjected to washing, which removes the CD ‘core’. Crucial points related to Step 1, 2 and 3 are discussed in the context of Fig. 1. | ||
| Fig. 1 Schematic diagram showing the three step process to fabricate the metal oxide tubes and the mechanism involved in the ALD process. | ||
Characterization
A scanning electron microscope (SEM, FEI-Quanta 200 FEG) was employed to investigate the morphology and dimensions of the samples before and after the washing step. Nominal 5 nm Au/Pd was sputtered on the samples prior to the observation under SEM. Average fiber diameter (AFD) was estimated from SEM images with samplings well above the statistically acceptable limit. Metal oxide tubes were subjected to transmission electron microscopy (TEM, FEI-Tecnai G2 F30) measurements where the sample was dispersed in ethanol and a tiny droplet was analyzed from a holey carbon coated TEM grid. X-ray diffraction (XRD) patterns were recorded in the range of 2θ = 10–90° using PANalytical X'Pert Pro Multi Purpose X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å). The ionic states of the constituent elements at the surface of the samples (400 μm spot size) were investigated by X-ray photoelectron spectroscopy (XPS, Thermoscientific K-Alpha, Al Kα radiation with hν = 1486.6 eV) with a flood gun charge neutralizer. For the core-level spectra, the pass energy and step size were set to 30 eV and 0.1 eV, respectively. Valence band (VB) spectra were also recorded with a pass energy of 30 eV in energy steps of 0.2 eV. Spectral deconvolutions of the XPS data were performed through Avantage software while considering the chemistry of the sample. Origin 8.5 has been employed to deconvolute the XRD peaks with Lorentzian function. In the case of XRD, number of peaks and their angular location were the initial values while other parameters are set as free until convergence.Results and discussion
As outlined in the introduction, electrospinning is a quite potential technique to produce polymeric and non-polymeric nanofibers (Fig. 1, Step 1). When a polymer is subjected to electrospinning, the overlap and entanglement of the polymeric chains is essential for the electrospinning process. Analogous to this, CD molecules form ‘aggregates’ which fulfil the essential criteria of electrospinning process. These aggregates are accelerated in the electric field and rapidly solidify while reaching the substrate in the form of nanofibers.13Basically in an ALD process gaseous precursors are injected with a carrier gas into a chamber (containing the substrate) at a predetermined flow rate in a sequential manner. Precursor molecules are first adsorbed on the surface and then eventually chemisorbed by the reactive surface sites, forming a monomolecular layer of that precursor. In the case of Al2O3, –CH3 ligands in the TMAl interact with the surface as shown in Fig. 1, Step 2 (top). Note that we did not show the O bridge bonds. The remnant methyl groups of TMAl react with the next pulse of H2O. The sequence of reaction mechanism is shown on the image (Fig. 1). Note that the –CH3 ligands may interact with some other active functional groups if –OH groups are not available. If not the growth process stops until the next pulse of precursor (H2O) arrives. In the case of CD no pre-treatment is applied in the ALD process, as the CD molecules have sufficient density of –OH groups. Each CD molecule consists of ∼20 and ∼9 –OH groups for HPβCD and MβCD, respectively. However, if the reaction requires –OH mediation and if the surface lacks it then a pre-treatment with water vapor is generally applied. Most importantly the chemisorption of TMAl has to take place as one monomolecular layer on the sample surface. The surface chemical functionality of CD is quite interesting when compared to polymers those were subjected to ALD processing, because of the surface –OH groups. For ZnO deposition, DEZn also reacts similarly with the H2O molecules as shown in Fig. 1, Step 2 (bottom). Initially DEZn molecules arrive at the surface and form a surface bond with the –OH groups. When H2O is pulsed into the chamber the second ethyl group forms C2H6 gas leaving –OH groups behind. The reaction mechanism is shown on the image (Fig. 1, bottom of Step 2). The surface functional groups of CD can be considered as an additional advantage in the ALD process.
The representative SEM images of pristine and ALD treated CD nanofibers (before and after washing step) are shown in Fig. 2. It was observed that ALD process did not cause any disruption to the nanofiber morphology. It is interesting to note that the AFD decreases when the core–shell nanofibers are subjected to calcination to remove the polymeric core while the percentage shrinkage depends on the type and morphology of the polymer.5 On contrary, for the case of washing, we may not expect a change in the AFD. Severe morphological changes can occur when the nanofibers are subjected to thermal treatments.3,5 Once the ‘core’ is washed the resulting tube-like structure is apparent for all cases. In Fig. 2d, h, f and j the metal oxide coating is thin enough to be electron-transparent as the tubes from the bottom layers are explicit. In Fig. 2f we show a selected region displaying their tube-like structures.
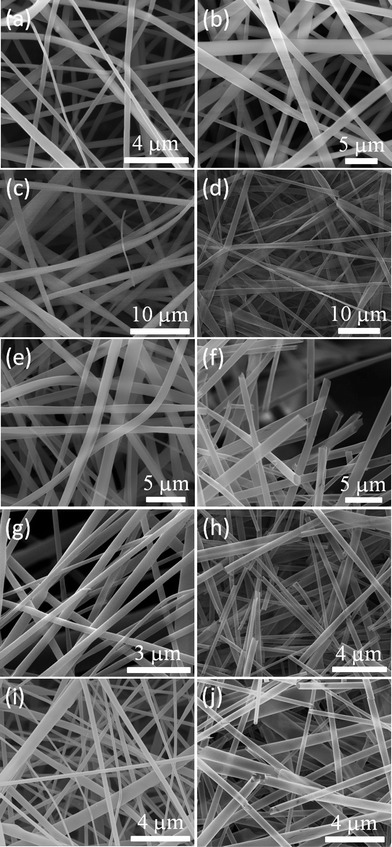 | ||
| Fig. 2 SEM images of samples before and after washing steps. (a) MβCD nanofibers, (b) HPβCD nanofibers (c) AM2, (d) AM2_W, (e) AH2, (f) AH2_W, (g) ZM2, (h) ZM2_W, (i) ZH4, and (j) ZH4_W. | ||
We have analyzed the diameters of samples after Al2O3 or ZnO coating for both the types of CD nanofibers. The results are plotted in Fig. 3 with Gaussian fittings. The center of the peak is numerically denoted on the corresponding graph. The process of fiber formation in electrospinning is quite complex and obtaining uniform morphology and diameter is a rather hard task.19–22 Similar to the case of polymeric fibers, the homogeneity and diameter of non-polymeric CD nanofibers highly depend on the used solvent types and concentration of the system.13,14 In our previous studies,13,14 the electrospinning parameters were optimized for both CD types to produce nanofibers having bead-free morphology, thus we were able to obtain uniform tube structures in this study. However there is a variance at the diameter distributions and this is because of the intrinsic nature of the electrospinning process. On the other hand, after the washing step the structural integrity of the nanofibers was preserved yielding metal oxide tubes.
Local crystal structure and the thickness of the shell coating can be precisely estimated by recording TEM images on the samples. We have used tube-form of samples for such measurements and typical images are shown in Fig. 4. 200 cycles of Al2O3 resulted in a thickness of ∼20 nm, 400 cycles of ZnO has developed a thickness of ∼40 nm. Samples AM2_W and AH2_W generally have a smoother morphology when compared to those of ZM4_W and ZH4_W samples. The smoother morphology is convincing as Al2O3 did not depict any grainy structure while in contrast ZnO has shown well developed grains. STEM (dark field) image is shown in Fig. 4b for AH4_W sample, where we explicitly see the tube-like structure. As expected ZM4_W and ZH4_W samples have shown well-developed crystal grains as seen in Fig. 4c and d for hollow, respectively. The grain boundaries can host oxygen-related functional groups, which are deterministic in the optical properties,23–25 photocatalysis,3–5 photoresponse23 etc. The electron diffraction pattern is shown for ZH4_W sample (inset of Fig. 4d) with the corresponding reflections annotated. These circular rings seen in the pattern consists of a series of dots representing the well-developed single crystals assembled in various directions. The pattern is found to be consistent with the literature.24,25
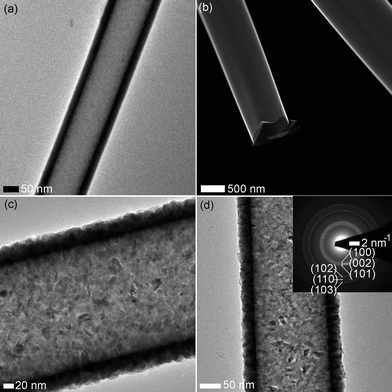 | ||
| Fig. 4 TEM of images of (a) AM2_W, (b) AH2_W, (c) ZM4_W, and (d) ZH4_W. The inset shows ED from ZH4. | ||
XRD patterns form ZM2, ZH4 and their washed counterparts are shown in Fig. 5. AH2, AM2 and their tube counterparts did not depict any clear diffraction peaks in the XRD analysis (not shown here). In contrast to Al2O3 case, explicit peaks were observed from ZnO and the corresponding reflections were annotated on Fig. 5. We have compared the characteristics (Table 1 of ESI†) of deconvoluted peaks (30–39°) for ZnO samples (ZM2, ZM2_W, ZH4, and ZH4_W) in terms of angular position and full width at half maximum (FWHM). Importantly, the values from Table 1 of ESI† suggest that the washing away the ‘core’ did not induce any strain in the lattice. As mentioned in the introduction, thermal treatment can also be employed to remove the ‘core’. In the case of calcination there can be a residual strain development, which is clearly not the case here. When the –OH groups of CD are randomly substituted with methoxy groups (MβCD) and hydroxypropyl (HPβCD), it results in amorphous material depicting a broad diffraction peak (2θ range not shown here) in contrast to pure β-CD which is a crystalline material.13,14
Ionic state of elements on the surface can be identified rather precisely by analyzing XPS spectra. It is important in various contexts, for example Al2O3 can be used as an anticorrosive coating, where a clear understanding of the surface chemical functionalities is required.26 While the surface characterization of the wide band gap material such as ZnO is of prime importance in the context of optical properties,23–25 photocatalysis,3–5 photoresponse23 etc. XPS survey scan (not shown here) is performed on all samples and the results from metal oxide tubes are tabulated in Table 2 of ESI.† Core level XPS spectra were analyzed on Al, Zn and O elements for all four cases before and after the washing step to infer any chemical changes occurred on the surface. Al2p spectra were shown in Fig. 6a and b from Al2O3 samples of AM2, AH2 and their hollow counterparts, respectively. A significant signal-to-noise ratio is obtained despite of the relatively low ionization cross-section of Al.27 The energetic values of Al2p doublet (2p3/2 and 2p1/2) are not only in line with literature but also comparable across the Al2O3 coated samples.28 However in the case of metal oxide tubes, a high energy component is seen for AM2_W (75.57 eV) and AH2_W (75.86 eV) cases which might be attributed to the surface bound hydroxyl groups on Al.28 The surface groups arose from ‘Step 3’ during the fabrication of tubes. In the case of ZnO, ZM2 and ZH4 samples and their tube counterparts did not depict any differences in the Zn2p core-level spectra (Fig. 1 of ESI†). The spectral locations of the peaks are in line with literature.25 Not observing any differences in Zn2p core-level spectra, of course, does not rule out the possibility of surface hydroxide formation or the presence of oxygen-related functional groups. We will see that this is the case in the context of O1s spectra from ZnO samples.
In the following we discuss the O1s core level spectra for Al2O3 and ZnO coatings from core–shell and metal oxide tubes. O1s spectra were shown in Fig. 7a and b from Al2O3 samples of AM2, AH2 and their tube counterparts, respectively. All the spectra were deconvoluted according to the chemistry of the material. The peak corresponding to lattice oxygen in each of the materials is shown as a shaded area in the plots. The spectra from AM2 an AH2 have shown major peaks centered at ∼531 eV corresponding to the oxygen in Al2O3 (OAl2O3) and are consistent with the literature.28 The other minor peak appeared at ∼529.5 eV for AM2 and AH2 samples, might be originated from contaminants while transporting the sample. The second peak on the higher binding energy (BE) side (∼533 eV) is attributed to adsorbed H2O (OH2O).4,5 Significant changes were observed for tube samples after ‘Step 3’, AM2_W and AH2_W where O1s spectra consist of four peaks (top parts of Fig. 7a and b). Importantly the peaks at ∼531 eV are from the OAl2O3 (shaded). While the peaks at ∼533 eV are from OH2O and those at ∼530 eV are from differential charging (Diff). Differential charging generally occurs on the surface because of the localized differences in the charge state.28 Especially the mixture of Al2O3 and AlOOH phases perhaps enhance such possibility. Also because of the washing step, prominent peak appeared around 532 eV corresponds to O in OH of AlOOH for AM2_W and AH2_W samples.28
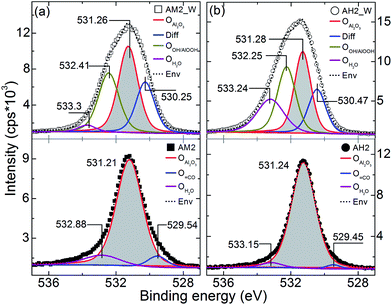 | ||
| Fig. 7 O1s core level XPS spectra from Al2O3 coated electrospun samples before and after washing steps, (a) AM2, AM2_W and (b) AH2, AH2_W. The peak positions are annotated on the plot in eV. | ||
O1s spectra from ZM2, ZH4 and their tube counterparts were shown in Fig. 8. All the spectra were deconvoluted into three peaks and the origin for each of them is discussed contextually. The peak corresponding to lattice oxygen from each of the materials is shaded in all cases. As mentioned earlier, the photocatalytic activity depends on the ionic state of the chemisorbed surface oxygen.3–5 O1 s spectra were shown in Fig. 8a and b from ZnO samples of ZM2, ZH4 and their tube counterparts, respectively. From Fig. 8a, the shaded peak within the range of 530.57–530.61 eV in all samples is attributed to O2− ions in the wurtzite structure which are supplemented by nearest neighbor Zn2+ in hexagonal configuration (OZnO). Apart from this, as prepared core–shell structures (ZM2 and ZH4) consist of two more peaks each on the higher BE side of OZnO. In the case of ZM2, 531.96 eV peak and 532.97 eV peak are attributed to chemisorbed oxygen, OCh1 and OCh2 respectively. ZH4 case, 532.03 and 533.35 eV peaks are ascribed to OCh1 and OCh2 respectively. Generally, the peak centered at 531.50 ± 0.1 eV is associated with Ox− ions (x < 2). The second high-energy minor peak at 532.50 ± 0.1 eV is typically ascribed to OH− groups, chemisorbed oxygen or dissociated oxygen.3–5 This higher BE peak (532.50 ± 0.1 eV) is also attributed to oxygen in –CO3 by some authors.27,29 It can be seen that the relative density of chemisorbed oxygen is higher for ZH4 than ZM2. The slight variation in the energy of the peak corresponding to non-native oxygen because what we see is an integral effect of various species. On the other hand, the grain boundaries (as seen in the TEM images) are obvious locations for such oxygen-related species to chemisorb. Since the sharing of an electron can be partial when combined with the above ions it has produced relatively broad binding energies peaked at ∼531.9 eV.
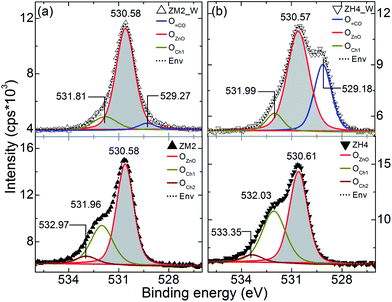 | ||
| Fig. 8 O1s core level XPS spectra from samples before and after washing steps, (a) ZM2, ZM2_W and (b) ZH4, ZH4_W. The peak positions are annotated on the plot in eV. | ||
Despite of the above discussed external oxygen content, the relative intensity of the peak corresponding to ZnO is the most prominent in all cases. The two non lattice origin peaks generally occur in connection to the oxygen vacancies (VOs) in the ZnO lattice.3–5,25 In other words, the relative intensities of these components can be connected to the variations in the density of VOs/chemical functionalities. When the core–shell nanofibers subjected to washing step to convert into metal oxide tubes, significant changes have taken place, see ZM2_W in Fig. 8a (top). ZM2_W has shown a component at 529.27 eV which is attributed to various contaminants. The second component ∼531.81 eV from ZM2_W is attributed to chemisorbed oxygen satisfying the earlier given argument. Furthermore, the surface-Zn(OH)2 component might be quite low when compared to the overall ZnO content. Note that the hydroxide formation is limited to the surface as we did not evidence any peaks corresponding to Zn(OH)2 in the XRD pattern (range not shown here).
Density of occupied VB states is investigated in the case of Al2O3 tubes and core–shell nanofibers and significant changes were observed before and after washing. The changes are limited to the features below the edge while the edge is undisturbed. A very high band gap (O2p to Al3s transitions) of Al2O3 arises because of predominant ionic natured bonding.30 Experimentally obtained VB density of states (DOS) was shown in Fig. 9. The upper VB consists of two main features (enclosed with three peaks) within the given energy range. O2s arises below the VB maximum around −20 eV (range not shown here). The first feature is nonbonding orbitals of O2p form the top of the upper VB (Fig. 9a). French30 suggests that these bands are ‘flat’ in k-space and correspond to localized state in real space forming O2− ion. Hence the VB is anionic in nature, in contrast empty Al antibonding orbitals corresponding to Al3+ form the cationic conduction band.30 The second feature corresponds to mixed Al and O origin. These are hybridized Al–O bonding orbitals (hAl–O) represent the covalent bonding present in Al2O3. Furthermore, above 14 eV a clear difference is seen for AH2 and AH2_W samples, where the former has shown relatively higher DOS in contrast to the latter. It is also notable that AH2_W is more comparable to the Al2O3 spectrum of native or thermal oxide.30 VB structure of ZnO is investigated and the results suggest that there are no changes in the VB edge before and after washing and MβCD to HPβCD nanofibers (spectra not shown here).
 | ||
| Fig. 9 Valance band spectra from Al2O3 coated samples before and after washing steps, (a) AM2, AM2_W and (b) AH2, AH2_W. | ||
We have attempted to obtain ZnO tubes with HPβCD nanofibers with 200 cycles of deposition. However after washing the tube structure is not sustained because of the following two reasons; (1) we have evidenced that HPβCD nanofibers are relatively thicker than MβCD nanofibers, (2) in the ALD process ZnO forms grain like structure. At higher diameter of core the grainy structure collapses due to the lack of mechanical robustness. Hence we have performed 400 cycles of deposition which has yielded structurally stable ZH4_W sample. Note that this is not the case with Al2O3 which has formed amorphous structure and hence 200 cycles of deposition is good enough to sustain the structure. Hence the crystalline nature of the ALD product determines the integrity of the structure.
Conclusions
In this study we show that non-polymeric systems such as CD (MβCD and HPβCD) are well compatible in ALD process. This combination can produce core–shell nanofibers and subsequently metal oxide tubes. CD nanofibers are compatible at relatively higher temperatures which can have a better applicability in ALD processing. Exploiting the excellent water solubility of CD we have tested two metal oxide coatings viz. Al2O3 and ZnO and thoroughly characterized the core–shell nanofibers and metal oxide tubes. By considering the deposition mechanism we note that the surface –OH groups of CD are beneficial to support a uniform coating. The surface morphology examination via SEM suggested smooth and consistent morphology within each of the samples. After washing the ‘core’ the tube-like structure is rather explicit under SEM. TEM images have evidenced smooth morphology for Al2O3 and grainy structure for ZnO. Electron diffraction confirmed the wurtzite structure of ZnO. XRD analyses of core–shell nanofibers and ZnO tubes corroborate no residual stress/strain development and wurtzite structure as well. XPS spectral analysis on Al2O3 and ZnO has confirmed the ionic state of Al and Zn. Core-level XPS on Al has shown a small fraction of hydroxylation. O1s from Al2O3 has shown significant variations within core–shell and tube samples. Noticeably tube samples host AlOOH on the surface apart from other typical functional groups. O1s from ZnO is discussed elaborately as the grainy structure and intrinsic defects can host a variety of functional groups. Similar to the case of O1s from Al2O, its VB structure also depicted notable differences which might arise from the formation of AlOOH on the surface because of the washing step.Acknowledgements
A.C. thanks The Scientific and Technological Research Council of Turkey (TUBITAK) TUBITAK-BIDEB-2228 for PhD scholarship. S.V. thanks TUBITAK (TUBITAK-BIDEB 2221-Fellowships for Visiting Scientists and Scientists on Sabbatical) for the postdoctoral fellowship). N.B. thanks EU FP7-Marie Curie-IRG for funding NEMSmart (PIRG05-GA-2009-249196). T.U. thanks EU FP7-Marie Curie-IRG (NANOWEB, PIRG06-GA-2009-256428) and The Turkish Academy of Sciences – Outstanding Young Scientists Award Program (TUBA-GEBIP) for partial funding. Authors thank M. Guler for technical support for TEM analysis.References
- W. J. Sweet, J. S. Jur and G. N. Parsons, J. Appl. Phys., 2013, 113, 194303 CrossRef PubMed.
- C. J. Oldham, B. Gong, J. C. Spagnola, J. S. Jur, K. J. Senecal, T. A. Godfrey and G. N. Parsons, J. Electrochem. Soc., 2011, 158, D549–D556 CrossRef CAS PubMed.
- F. Kayaci, S. Vempati, C. O. Akgun, N. Biyikli and T. Uyar, Appl. Catal., B, 2014, 156–157, 173–183 CrossRef CAS PubMed.
- F. Kayaci, S. Vempati, I. Donmez, N. Biyikli and T. Uyar, Nanoscale, 2014, 6, 10224–10234 RSC.
- F. Kayaci, S. Vempati, C. Ozgit, I. Donmez, N. Biyikli and T. Uyar, Nanoscale, 2014, 6, 5735 RSC.
- P. Heikkilä, T. Hirvikorpi, H. Hilden, J. Sievänen, L. Hyvärinen, A. Harlin and M. Vähä-Nissi, J. Mater. Sci., 2012, 47, 3607–3612 CrossRef.
- Q. Peng, X.-Y. Sun, J. C. Spagnola, G. K. Hyde, R. J. Spontak and G. N. Parsons, Nano Lett., 2007, 7, 719–722 CrossRef CAS PubMed.
- Q. Peng, X.-Y. Sun, J. C. Spagnola, C. Saquing, S. A. Khan, R. J. Spontak and G. N. Parsons, ACS Nano, 2009, 3, 546–554 CrossRef CAS PubMed.
- J. Y. Park, S.-W. Choi and S. S. Kim, Nanotechnology, 2010, 21, 475601 CrossRef PubMed.
- S. Cho, D.-H. Kim, B.-S. Lee, J. Jung, W.-R. Yu, S.-H. Hong and S. Lee, Sens. Actuators, B, 2012, 162, 300–306 CrossRef CAS PubMed.
- E. Santala, M. Kemmel, M. Leskela and M. Ritala, Nanotechnology, 2009, 20, 035602 CrossRef PubMed.
- F. Kayaci, C. Ozgit-Akgun, N. Biyikli and T. Uyar, RSC Adv., 2013, 3, 6817–6820 RSC.
- A. Celebioglu and T. Uyar, Chem. Commun., 2010, 46, 6903–6905 RSC.
- A. Celebioglu and T. Uyar, Nanoscale, 2012, 4, 621–631 RSC.
- A. Celebioglu and T. Uyar, J. Colloid Interface Sci., 2013, 404, , 1–7 CrossRef PubMed.
- A. Celebioglu and T. Uyar, RSC Adv., 2013, 3, 22891–22895 RSC.
- M. G. McKee, J. M. Layman, M. P. Cashion and T. E. Long, Science, 2006, 311, 353–355 CrossRef CAS PubMed.
- X. Yan, M. Zhou, J. Chen, X. Chi, S. Dong, M. Zhang, X. Ding, Y. Yu, S. Shaod and F. Huang, Chem. Commun., 2011, 47, 7086 RSC.
- S. Ramakrishna, K. Fujihara, W. Teo, T. Lim and Z. Ma, An Introduction to Electrospinning and Nanofibers, World Scientific Publishing Company, Singapore, 2005 Search PubMed.
- J. H. Wendorff, S. Agarwal and A. Greiner, Electrospinning: Materials, Processing, and Applications, Wiley-VCH, Germany, 2012 Search PubMed.
- J. V. Nygaard, T. Uyar, M. Chen, P. Cloetens, P. Kingshott and F. Besenbacher, Nanoscale, 2011, 3, 3594–3597 RSC.
- T. Uyar and F. Besenbacher, Polymer, 2008, 49, 5336–5343 CrossRef CAS PubMed.
- S. Vempati, S. Chirakkara, J. Mitra, P. Dawson, K. K. Nanda and S. B. Krupanidhi, Appl. Phys. Lett., 2012, 100, 162104 CrossRef PubMed.
- S. Vempati, J. Mitra and P. Dawson, Nanoscale Res. Lett., 2012, 7, 470 CrossRef PubMed.
- S. Vempati, A. Shetty, P. Dawson, K. K. Nanda and S. B. Krupanidhi, Thin Solid Films, 2012, 524, 137 CrossRef CAS PubMed.
- B. Díaz, E. Härkönen, J. Swiatowska, V. Maurice, A. Seyeux, P. Marcus and M. Ritala, Corros. Sci., 2011, 53, 2168–2175 CrossRef PubMed.
- M. Chen, X. Wang, Y. H. Yu, Z. L. Pei, X. D. Bai, C. Sun, R. F. Huang and L. S. Wen, Appl. Surf. Sci., 2000, 158, 134–140 CrossRef CAS.
- B. V. Crist, Handbooks of Monochromatic XPS Spectra: Commercially Pure Binary Oxides, XPS International, California, 2005, http://http//:www.xpsdata.com Search PubMed.
- S. Major, S. Kumar, M. Bhatnagar and K. L. Chopra, Appl. Phys. Lett., 1986, 49, 394 CrossRef CAS PubMed.
- R. H. French, J. Am. Ceram. Soc., 1990, 73, 477–489 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Output results of XRD peak deconvolution, results of XPS survey scan and Zn2p core-level XPS. See DOI: 10.1039/c4ra12073f |
| This journal is © The Royal Society of Chemistry 2014 |



