Application of the polyacrylonitrile fiber as a support for the green heterogeneous base catalyst and supported phase-transfer catalyst†
Abstract
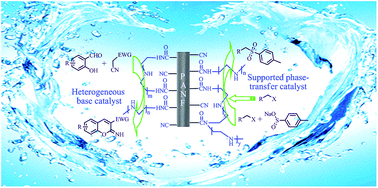
A facile synthesized polyethylene polyamine functionalized polyacrylonitrile fiber (PANF-PA), capable of acting as the heterogeneous base catalyst and supported phase-transfer catalyst (SPTC), was presented in Knoevenagel condensation–cyclization and nucleophilic substitution for the synthesis of a number of substituted iminocoumarins and sulfones in aqueous systems, respectively. Excellent results are shown in terms of simple procedures, high chemical yields (up to 96%), extensive applicability and superior catalytic recyclability even for 10 cycles. The deeper active principle of the PANF-PA as SPTC was explained through detailed characterization by means of elemental analysis, FTIR spectroscopy and SEM. Moreover, the procedures can be effectively scaled up, and the solvent can be easily recovered. As well as, the prominent features (high strength, good flexibility etc.) of the polyacrylonitrile fiber are very attractive for fixed-bed reactors in the chemical industry.

 Please wait while we load your content...
Please wait while we load your content...