3D chemically cross-linked single-walled carbon nanotube buckypapers†
Michael B. Jakubineka,
Behnam Ashrafib,
Jingwen Guana,
Michel B. Johnsonc,
Mary Anne Whitecd and
Benoit Simard*a
aSecurity and Disruptive Technologies Portfolio, Division of Emerging Technologies, National Research Council Canada, Ottawa, Canada K1A 0R6. E-mail: Benoit.Simard@nrc-cnrc.gc.ca
bAerospace Portfolio, Division of Engineering, National Research Council Canada, Montreal, Canada H3T 2B2
cInstitute for Research in Materials, Dalhousie University, Halifax, Canada B3H 4R2
dDepartment of Chemistry, Dalhousie University, Halifax, Canada B3H 4R2
First published on 4th November 2014
Abstract
Single-walled carbon nanotubes (SWCNTs) covalently modified with OH functional groups were assembled into buckypapers through solvent dispersion and vacuum filtration. These SWCNT-OH buckypaper sheets were subsequently crosslinked by wetting with bifunctional linkers followed by hot compression causing reaction between the functional groups of the reagent and OH functional groups on the side-walls of SWCNTs to create three-dimensional (3D) covalently cross-linked buckypapers. Cross-linking also was performed using SWCNTs encapsulated with a functionalized polymer wrapping in a core–shell structure, where OH or/and NH2 groups are available on the surface of the polymeric shell for reaction. The 3D cross-linked SWCNT buckypapers retain the porous character typical of buckypaper, and were characterized for their tensile properties and thermal and electrical conductivities. Several cross-linking approaches dramatically improved the mechanical properties. The strongest and stiffest papers (32 MPa, E = 3.1 GPa), which approach 10× stronger and stiffer than the pristine non-crosslinked buckypaper, were obtained at the expense of a loss of electrical conductivity. In other cases, such as cross-linking using a high-performance epoxy resin monomer, improvements in strength and stiffness of ∼5× were obtained while retaining electrical and thermal conductivity. Therefore, the optimal cross-linking approach would be determined by the desired, multifunctional properties. Additionally, the approach can be used in the preparation buckypaper composites and it is demonstrated that cross-linking using a multifunctional epoxy resin prior to impregnation with the same epoxy resin results in substantially better mechanical properties in comparison to just epoxy-impregnation of pristine buckypaper.
Introduction
Growth or fabrication of macroscopic assemblies composed of carbon nanotubes (CNTs), such as papers, arrays and yarns, is a promising approach to harness the outstanding properties of CNTs for development of new engineering materials, in part due to higher CNT volume fractions achievable in assemblies compared with randomly dispersed CNT composites. Non-woven sheets of carbon nanotube paper were the first such large-scale CNT assemblies.1 Often called buckypaper, these CNT sheets are most commonly formed by vacuum filtration of a suspension of randomly distributed and oriented CNTs. Buckypaper sheets have garnered interest for mechanical reinforcement in composites2–8 as well as a wide range of multifunctional applications including fire retardancy,9 electromagnetic shielding,10 thermal interface materials,11,12 actuators,13,14 sensors,15 gas separators,16 field emitters,17 and electrodes for fuel cells,18 supercapacitors,19,20 and batteries.21,22Buckypaper performance in many applications is largely influenced by its mechanical properties, whether directly for structural applications or indirectly wherein the robustness and flexibility of the paper is crucial to maintain the sheet integrity during processing, integration, manufacturing and use for multifunctional applications. The mechanical properties of pristine buckypaper sheets are often low as the papers are typically highly porous (∼60–75 vol% void) and the interaction between adjacent CNTs and CNT bundles is through van der Waals forces. For example, even in a bundle where the CNTs are perfectly aligned, the strength of the assembly is limited by CNT–CNT sliding over a wide range of CNT aspect ratio.23,24 As a consequence, the elastic modulus (E) and ultimate tensile strength (UTS) of randomly oriented CNT buckypapers are often in the range of 0.1–1 GPa and 1–10 MPa, respectively,25–28 which is only a small fraction of individual CNTs (E ∼ 1 TPa, UTS ∼ 100 GPa).
Resin impregnation significantly improves the mechanical properties of buckypaper; however, it changes the buckypaper structure by filling air voids, changing it from a permeable to a non-permeable film, increasing density, and reducing the electrical conductivity. As a result, for multifunctional applications it can be desirable to improve mechanical properties without substantially impregnating the buckypaper. CNT aspect ratio, alignment, and volume fraction, as well as cross-linking between adjacent CNTs, all factor into the overall mechanical properties of neat buckypaper sheets. While it has been shown that increasing CNT alignment and aspect ratio can increase the mechanical properties due to an increase of fiber-to-fiber friction and entanglement,29 the main limiting factor for the mechanical properties is the interaction between adjacent CNTs or bundles. From a practical standpoint, higher aspect ratio CNTs also are more challenging to disperse in solvents, limiting their applicability for production of buckypaper by some of the common methods (e.g., filtration). A theoretical model based on joint morphology developed to predict the upper-bound moduli of buckypapers containing nanotube ropes suggests that strong joints (no sliding) between CNT interconnects can increase the elastic modulus to 1% to 10% of the bundle elastic modulus.26 This information suggests the possibility of a 2- to 15-fold increase in elastic modulus for buckypaper (versus the best experimental data) if the volume fraction and connection between CNTs are improved. A buckypaper in which the nanotubes are covalently cross-linked within and between bundles provides a possible embodiment of this structure, where covalent bonds would provide stronger CNT–CNT interaction and resistance to sliding.
Both experimental and theoretical studies have shown that mechanical properties can be improved through irradiation with electrons or ions to induce covalent cross-links.30–34 In these approaches, irradiation causes defects that include covalent cross-links, interstitial atoms confined between CNTs, or even coalescence of CNTs. Significant increases (∼50%) in tensile strength were recently reported following electron irradiation of CNT sheets.35 Chemical methods to improve CNT–CNT interaction based on functionalized CNTs also have been reported. For example, tensile strengths up to twice that of pristine buckypaper were achieved by functionalization with a chain containing multiple amide groups that hydrogen bond to increase the strength of CNT–CNT interaction.36 Covalent cross-linking of NH2-functionalized MWCNT buckypapers with a benzoquinone solution has been reported to increase tensile strength and simultaneously improve electrical conductivity.37 Thiol-functionalized MWCNTs also have been cross-linked with benzoquinone.38
In this paper we report several variations of CNT–CNT cross-linking based primarily on infiltration of hydroxyl-functionalized single-walled carbon nanotube (SWCNT-OH) buckypapers with bi- (or tri)-functional reagents to create 3D covalently cross-linked SWCNT buckypapers. Buckypapers prepared using polymer coated SWCNTs were similarly cross-linked via surface functional groups (–OH and –NH2) on the polymer shell. In both cases the approach can be applied with a wide range of reagents provided there are two or more functional groups available for reaction and either the viscosity is sufficiently low to infiltrate the buckypaper or the reagent can be diluted/dissolved with solvent for infiltration. The cross-linked buckypapers were assessed in terms of their mechanical, thermal, and electrical properties in comparison to pristine SWCNT buckypaper. Selected cross-linking methods were found to substantially improve mechanical properties; furthermore, it was possible to produce stronger papers that retained their multifunctional character, and a combination of epoxy-mediated cross-linking followed by epoxy-impregnation provided substantially better mechanical properties than impregnation alone.
Methods
Preparation of pristine SWCNT (P-SWCNT) buckypaper
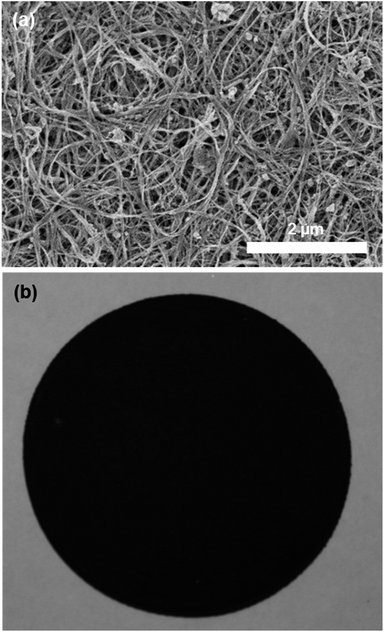
SWCNTs were produced by a variation of the laser ablation method39 and purified by an in-house wet chemistry purification protocol.40,41 P-SWCNT buckypapers (Fig. 1) were then produced by a binder-free, filtration method similar to previous reports for SWCNT buckypaper.7,28 Briefly, the purified SWCNTs were dispersed in methanol using a combination of bath (Fisher Scientific FS110) and probe (Branson Sonifier 250, 30% output, 50% duty cycle) sonication, followed by vacuum filtering a controlled volume of suspension through a polycarbonate filter membrane (Sterlitech, 47 mm diameter, 20 μm pore size) and drying under compression before peeling off the circular buckypaper. Bath sonication was applied immediately before pouring the suspension onto the filter to maintain the dispersion and uniform concentration.Preparation of SWCNT-OH buckypapers
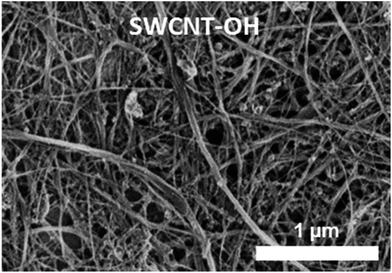
Based on the literature procedures for –OH functionalization of CNTs,42,43 800 mg of purified SWCNTs were ground in HCCl3 and transferred into 500 ml Schlenk flask and bath-sonicated in 450 ml of dry HCCl3 until well dispersed. The suspension was degassed with argon and, under argon, 45.6 g anhydrous AlCl3 powder was added, and mixed with a magnetic stir bar for a few minutes, followed by bath sonication for 1.5 h. The mixture was then refluxed at 80 °C for 4 h, then at 60 °C overnight (∼15 h), and the temperature was then held at 85 °C for another 6 h. Finally, the mixture was cooled to room temperature. A concentrated KOH solution in MeOH (50 g KOH/140 ml MeOH) was added dropwise (caution: exothermic!) to the mixture under strong stirring in an ice bath. The mixture was stirred overnight and refluxed for 8 h. After cooling to room temperature, the mixture was slowly diluted with distilled water, and the SWCNTs functionalized with –CH2OH groups (abbreviated to SWCNT-OH to identify the group available for further reaction) were suspended within the top water layer. Most of the yellow layer of CHCl3 at the bottom was removed through a separatory funnel. Hexane was added to the residue and, after shaking, SWCNTs gathered at the interface of hexane and water, and the remaining chloroform and most of the water was drained away. The mixture was further washed with distilled water. Finally, the residue mixture in the funnel was filtered through a polycarbonate membrane and extensively washed with distilled water and methanol before collecting the SWCNT-OH. The sample was dried in air, followed by oven-drying at 100 °C. Using this SWCNT-OH material, SWCNT-OH buckypapers were prepared in the same way as the P-SWCNT buckypapers described above. The SWCNT-OH buckypaper looks macroscopically identical to the pristine SWCNT paper, although density measurements and SEM micrographs (e.g., Fig. 2) both show a more porous structure.3D-cross-linking of SWCNT-OH buckypapers
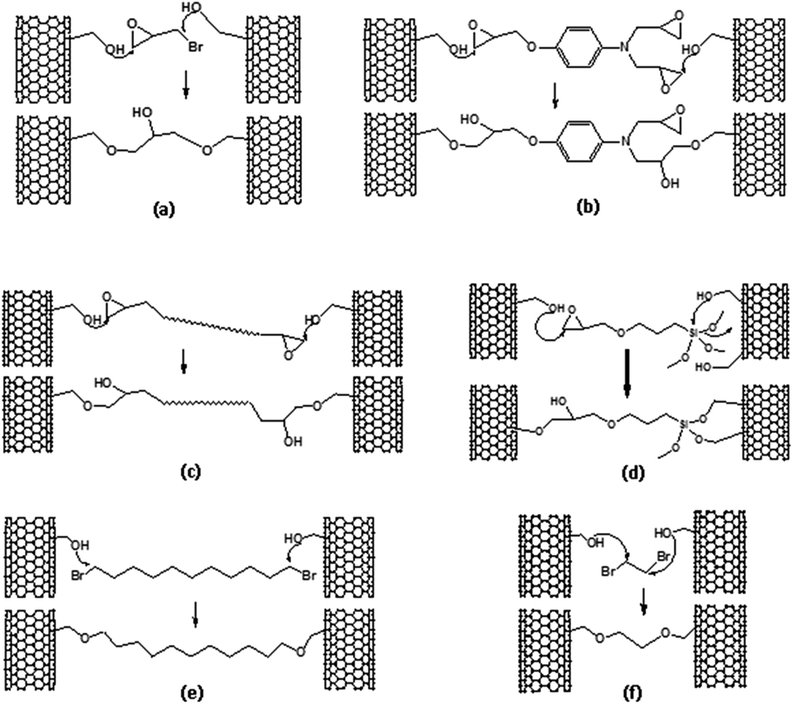
The SWCNT-OH buckypaper provided a common starting point for production of 3D-networking via inter-tube and inter-bundle cross-links. The general protocol consisted of: (1) preparation of SWCNT-OH buckypapers, (2) wetting SWCNT-OH buckypaper with a cross-linking reagent with or without solvent dilution, (3) hot-compression at 100 °C for 2 h and (4) slow cooling to room temperature (overnight) while under compression. Addition of solvent only serves to dilute the reagents to obtain uniform wetting of the buckypaper, which is particularly important for higher viscosity reagents. The 3D-cross-linked buckypapers produced are listed in Table 1 and labeled based on the interconnecting reagent (Int.-A to Int.-F) employed for cross-linking. The chemical reactions are shown schematically in Fig. 3.3D-cross-linked SWCNT-core–shell buckypapers
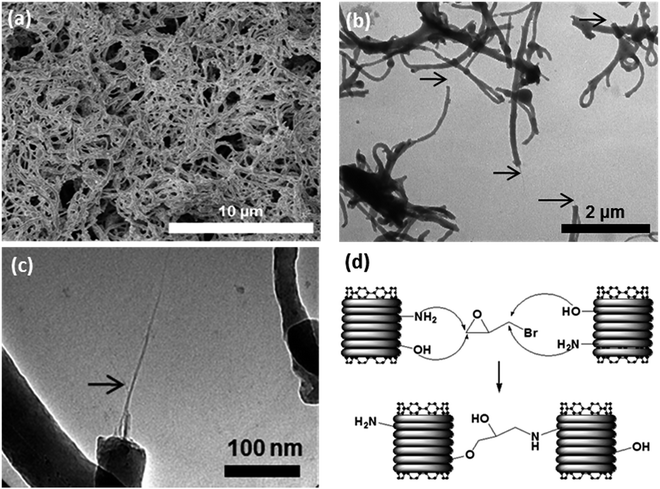
In addition to the SWCNT-OH approach, cross-linked buckypaper also was prepared by reaction of SWCNT-core–shell buckypapers containing the surface functional –NH2 and –OH groups from the polymeric shell on a single tube or a small bundle with external linkers, in the same way as for SWCNT-OH buckypaper. The SWCNT-core–shell structures (Fig. 4) were first prepared through a diazonium reaction between SWCNTs and 1,4-vinylaniline followed by in situ polymerization as described elsewhere.44 The cross-linking reactions for such a core–shell structure with epibromohydrin are shown schematically in Fig. 4d. Similar to the SWCNT-OH case, the epibromohydrin was diluted in hexane and the reaction took place under hot compression, in this case at 80 °C for 2 h and at 100 °C for an additional 2 h followed by slow cooling to room temperature overnight under compression.Characterization
The control and cross-linked buckypapers were characterized by scanning electron microscopy (SEM; Hitachi S4700) and thermogravimetric analysis (TGA). TGA was performed in both oxidation and desorption modes using a Netzsch TG 209 F1 Iris. The system was run with BOC UHP argon (5.3) gas for desorption; residual oxygen was trapped with a Supelco Big-Supelpure O oxygen/water trap. The elevated temperature runs were from room temperature to 950 °C at 10 °C min−1.Uniaxial tensile tests were performed on the buckypapers using a micro-tensile test frame (Fullam Substage Test Frame) at displacement rate of 1 mm min−1. A load cell with a capacity of 50 N and an extensometer attached to the moving grip were used for force and displacement measurements, respectively. Strips 1–2 mm wide and 20–30 mm long were tested to compare the tensile properties of the pristine SWCNT buckypaper, SWCNT-OH buckypaper, and 3D-cross-linked buckypapers. Elastic modulus (E), ultimate tensile strength (UTS), toughness (G) and strain at failure (εmax) were determined from the stress–strain curves. Density of the buckypapers was also calculated from the mass, diameter and thickness of the approximately 35 mm diameter papers before cutting into smaller samples for testing.
Sheet resistance (Rs) of buckypaper samples approximately 1 cm × 1 cm was measured at room temperature using the van der Pauw method. Electrical conductivity (σ) was calculated from the measured Rs and the sample thickness (t), σ = 1/(Rst). Four contacts to the sample were made by applying pressure with tungsten probes using a probe station (Cascade Microtech). Independent Keithley 2400 source-measure units were used as the current source and voltmeter, respectively. A switching box was used to automatically rotate the current source and voltmeter through all four contacts to the sample, thereby employing forward and reverse currents and symmetry to eliminate offset voltages and optimize the accuracy of the measurement. Multiple measurements using different test currents were performed to ensure that the result was both reproducible and negligibly subject to Joule heating.
Thermal conductivity (κ) was measured using the parallel thermal conductance (PTC) technique,45 as previously implemented for the measurement of CNT yarns and sheets.46,47 The PTC technique is well suited to the measurement of buckypaper, as it does not require the sample to support a heater and thermometers. The PTC method is a 1D steady-state measurement but differs from the archetypal longitudinal steady-state method, because the background conductance of the support is substantial and must be precisely determined and subtracted. Three PTC measurements (background, sample and radiation correction) were conducted to determine κ for each sample, and two samples of each type of buckypaper were measured. The measurements were performed at 300 K and under vacuum (10−5 Torr).
Results and discussion
Modified buckypapers
SEM images of the cross-linked buckypapers derived from SWCNT-OH paper are shown in Fig. 5. In some cases such as the epoxy-cross-linked buckypapers there is a change in the surface topology, with less porosity evident, although not to the same degree seen for an epoxy-impregnated buckypaper. This shows that the buckypapers remain porous and have not been substantially impregnated by the addition of the selected cross-linking reagents. For epibromohydrin-cross-linked buckypapers the topology closely resembles the control (pristine and SWCNT-OH buckypapers). The buckypapers derived from the core–shell SWCNT structure have a similar, non-woven fiber, appearance, as shown in Fig. 6, but with large-diameter fibers due to the larger diameter SWCNT–polymer core shell structure. The difference in composition of the core–shell SWCNT structure also is indicated by X-ray photoelectron spectroscopy, which shows increased oxygen and nitrogen contents of 9 atomic% and 7 atomic%, respectively, in comparison to <5 atomic% oxygen and <0.5 atomic% nitrogen, respectively, for the pristine SWCNTs.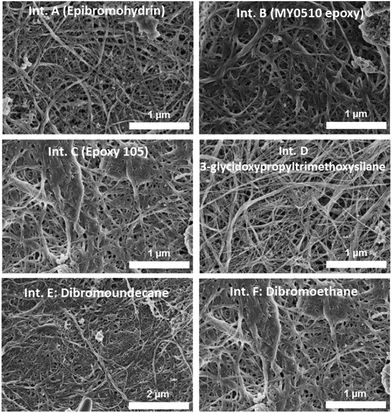 | ||
| Fig. 5 SEM images of cross-linked buckypapers derived from SWCNT-OH buckypaper using various cross-linking agents. | ||
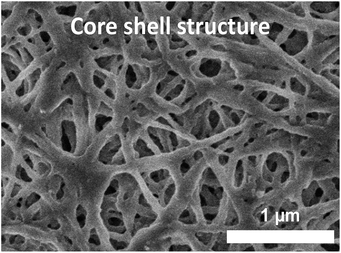 | ||
| Fig. 6 SEM images of cross-linked buckypapers derived from an SWCNT-core–shell structure buckypaper. | ||
The buckypapers were also characterized by TGA. The thermograms for pristine and SWCNT-OH buckypapers in both oxidation and desorption modes are similar in appearance (Fig. 7), due to the small change in composition. However, there is a clear shift to lower SWCNT oxidation temperature for the SWCNT-OH case, which is attributed to interruptions in the sp2 structure associated with functionalization (i.e., defects). TGA in desorption mode can be used to estimate degree of functionalization.48,49 A larger mass loss in desorption was seen for the SWCNT-OH paper, which again is consistent with the functionalization chemistry employed. Quantitative analysis of functionalization from TGA has a high uncertainty for small functional groups (in this case CH2OH) at typical degrees of functionalization of a few percent. For example, if the result is adjusted to account for the fact that there is already a ∼5% mass loss for the pristine buckypaper (instead of assuming the entire desorption mass-loss [∼10%] is due to –OH functionalization), and there is a metal impurity indicated by the residual mass (metal oxide) after high temperature oxidation, then the degree of functionalization estimate is cut in half. However, consideration of both cases gives an estimate of degree of functionalization in the range of 2–4 carbon% (2–4 CH2OH groups per 100 carbons) and consistent with other estimates for CNT functionalization.
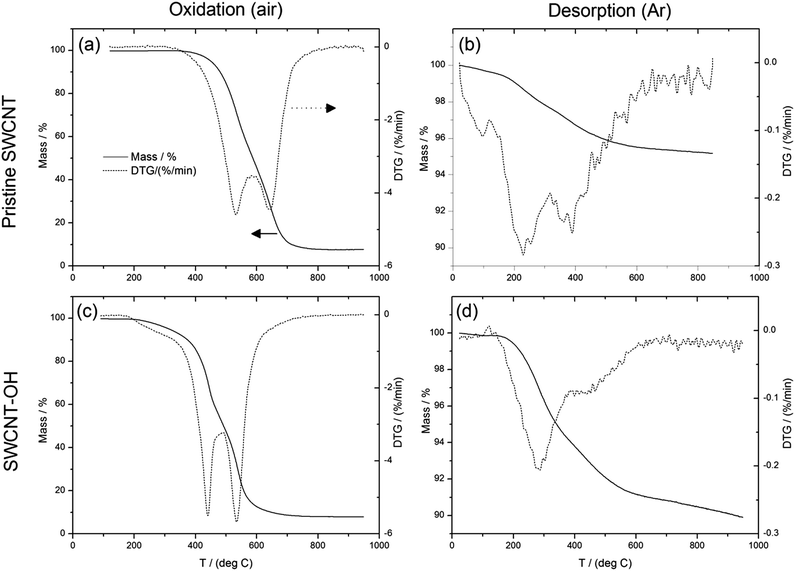 | ||
| Fig. 7 TGA of (a and b) pristine SWCNT and (c and d) SWCNT-OH buckypapers in oxidation and desorption modes. | ||
The residual masses for the baseline and cross-linked buckypapers derived from SWCNT-OH paper are summarized in Table 2 (see ESI, Fig. S1,† for the thermograms). Increased desorption mass loss (i.e., decreased residual mass) is due to the addition of the cross-linking reagent. Changes in the mass loss events (number of events and their temperatures) in comparison to the SWCNT-OH paper, which was the control for the cross-linking cases, indicate that reaction had occurred. However, in some cases lower temperature oxidation and desorption events were observed, which could be explained by the remaining unreacted crosslinking reagent within the paper. The residue after desorption also has been oxidized, which gives TGA features similar to the control, pristine buckypaper.
| ID | Cross-linking reagent | Oxidation (%) | Desorption (%) |
|---|---|---|---|
| P-SWCNT | NA | 8 | 95 |
| SWCNT-OH | NA | 8.2 | 90 |
| Int.-A | Epibromohydrin | 7.6 | 77 |
| Int.-B | MY0510 epoxy | 2.2 | 67 |
| Int.-C | Epoxy-105 | 3.2 | 67 |
| Int.-D | 3-Glycidoxypropyl-trimethoxysilane | 14.7 | 67 |
| Int.-E | 1,11-Dibromoundodecane | 6.7 | 64 |
| Int.-F | 1,2-Dibromoethane | 7.9 | 79 |
Properties of cross-linked buckypapers
The tensile properties, density, and electrical and thermal conductivities of the P-SWCNT, SWCNT-OH and 3D-cross-linked buckypapers are reported in Table 3. To facilitate comparison of a wide range of values, Fig. 8 shows each property relative to its corresponding value for pristine SWCNT buckypaper (e.g., E/EP-SWCNT buckypaper). Thermal conductivity differences between buckypapers were small and, due to measuring a smaller number of samples per buckypaper (two small samples from different sections of the original pressed buckypaper), could be masked by variations in thickness within an individual buckypaper. As a result the reported thermal conductivities have been scaled by the ratio of the average density of the buckypaper to the density of the smaller strip for which κ was measured. This was not necessary for other properties due to greater variation between different buckypapers and larger sample size and/or number of samples.| Buckypaper | Ultimate strain | UTS (MPa) | E (MPa) | Yield strength (MPa) | Toughness (J mm) | Density (g cm−3) | σ (S cm−1) | κ (W m−1 K−1) |
|---|---|---|---|---|---|---|---|---|
| P-SWCNT | 0.016 ± 0.010 | 3.4 ± 0.9 | 370 ± 90 | 2.4 ± 0.3 | 0.037 ± 0.030 | 0.42 ± 0.06 | 117 ± 6 | 2.4 ± 0.3 |
| SWCNT-OH | 0.007 ± 0.004 | 3 ± 2 | 400 ± 230 | 2.4 ± 1.7 | 0.014 ± 0.015 | 0.27 ± 0.04 | 45 ± 2 | 1.1 ± 0.2 |
| Int.-A | 0.012 ± 0.004 | 9 ± 2 | 1220 ± 80 | 6.7 ± 0.6 | 0.071 ± 0.044 | 0.50 ± 0.08 | 113 ± 6 | 2.7 ± 0.3 |
| Int.-B | 0.013 ± 0.004 | 15 ± 2 | 2060 ± 210 | 11.1 ± 0.4 | 0.120 ± 0.052 | 0.69 ± 0.10 | 46 ± 2 | 3.4 ± 0.2 |
| Int.-C | 0.011 ± 0.005 | 6 ± 3 | 890 ± 340 | 4.2 ± 1.9 | 0.050 ± 0.038 | 0.54 ± 0.08 | 40 ± 2 | 3.2 ± 0.3 |
| Int.-D | 0.011 ± 0.004 | 8.7 ± 1.5 | 1060 ± 250 | 6.3 ± 1.2 | 0.060 ± 0.032 | 0.43 ± 0.06 | 64 ± 6 | 1.9 ± 0.2 |
| Int.-E | 0.020 ± 0.004 | 3.0 ± 0.9 | 230 ± 80 | 2.2 ± 0.4 | 0.036 ± 0.019 | 0.60 ± 0.09 | 60 ± 3 | 3.1 ± 0.3 |
| Int.-F | 0.0041 ± 0.0010 | 1.9 ± 0.5 | 430 ± 20 | 1.9 ± 0.5 | 0.005 ± 0.003 | 0.30 ± 0.05 | 21 ± 2 | 1.5 ± 0.2 |
| Int.-core shell | 0.0112 ± 0.0014 | 32 ± 4 | 3060 ± 190 | 29 ± 4 | 0.202 ± 0.051 | 0.68 ± 0.10 | — | 1.9 ± 0.2 |
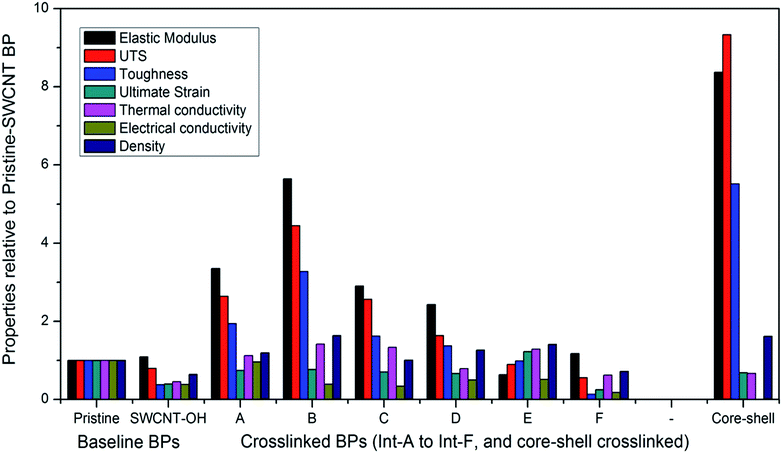 | ||
| Fig. 8 Properties of SWCNT-OH buckypaper and cross-linked buckypapers (refer to Table 1 for legend) relative to pristine SWCNT buckypaper (color online). | ||
With the exception of its stiffness, SWCNT-OH buckypaper has reduced properties in comparison to P-SWCNT buckypaper; however, much of this difference is accounted for by its lower density (∼65% of that for P-SWCNT buckypaper) and both the strength and stiffness of SWCNT-OH buckypaper are actually higher than P-SWCNT buckypaper on a per mass basis. With the exception of Int.-E, all cross-linked buckypapers have lower ultimate strain, attributed to an inhibiting effect of the cross-links on CNT-CNT sliding. Int.-E uses a long alkane chain (1,11-dibromoundecane), which would have less effect on CNT–CNT sliding. This is particularly evident in comparison of Int.-E to Int.-F, which uses the shortest dibromoalkane chain (1,2-dibromoethane). The shorter cross-link for Int.-F results in roughly twice the stiffness but provides the smallest maximum elongation of all the cross-linked cases tested. While the cross-linking with dibromo-alkanes was ineffective for improving properties, cross-linking using epoxide groups for one or both links (Int. A–D) provided improved strength, stiffness and toughness in comparison to P-SWCNT buckypaper. In addition to the covalent bonds illustrated in Fig. 3, these cross-linkers provide potential for additional binding via hydrogen bonding of –OH groups or, in cases B and D, an additional covalent bond to the SWCNT via epoxide or silane centers (e.g., two bonds to the right hand SWCNT are illustrated in Fig. 3d). Epoxies are generally known for their good mechanical properties and the best performance for the cross-linked buckypapers based on SWCNT-OH was achieved using MY0510 epoxy, which is a high performance aerospace epoxy resin, as an interlinking molecule. Stiffness of the crosslink might also relate to the improved thermal conductivity, with stiffer connections facilitating inter-CNT phonon transport. However, the increased density (i.e., reduced porosity) of the buckypapers caused by cross-linking can account for most of the observed thermal conductivity differences.
The stiffest (>8× P-SWCNT buckypaper), strongest (∼10× P-SWCNT buckypaper) and toughest (∼6× P-SWCNT buckypaper) buckypaper was made by cross-linking between SWCNT–polymer core shell structures via reaction with epibromohydrin (Fig. 4). This case is in some sense intermediate between a buckypaper and a SWCNT–polymer composite, as the SWCNTs are polymer-coated and the density is higher than that of buckypaper but the less than that of a polymer or typical SWCNT/polymer composite, and the structure remains porous (see SEM in Fig. 6). This approach results in better tensile properties but greatly reduces electrical conductivity.
In contrast to tensile properties and thermal conductivity, electrical conductivity was generally reduced in the cross-linked SWCNTs buckypapers. This fact is attributed in part to disrupting the SWCNT structure through covalent bonding, as seen from the lower conductivity of SWCNT-OH buckypaper. Even when adjusted for its lower density, SWCNT-OH buckypaper has only 60% of the conductivity of P-SWCNT buckypaper. Electrical conductivity is similarly low in most of the cross-linked buckypapers but does reach the value of P-SWCNT buckypaper for the Int.-A case. In this case, epibromohydrin forms a good mechanical interconnection, keeping the nanotubes in close contact and increasing density of the buckypaper significantly relative to the SWCNT-OH buckypaper. Density is also increased in most other cross-linked buckypapers but the longer interconnections could have a more significant influence on CNT–CNT resistance. The epibromohydrin cross-linked buckypapers, or other cases such as epoxy resin crosslinks where a significant portion of the electrical conductivity is retained, provide a multifunctional buckypaper due to maintained conductivity and improved (>2×) stiffness, strength, and toughness relative to P-SWCNT buckypaper.
Buckypaper–epoxy composites
Cross-linked buckypapers can be a starting point for further processing and integration into composite structures (e.g., impregnation, lamination). The Int.-B case (via MY0150 epoxy resin) is of particular interest as an example of a buckypaper–epoxy composite. In this case both P-SWCNT and cross-linked SWCNT buckypapers based on MY0510 (i.e., Int. B) were impregnated with MY0510 resin. The epoxy for impregnation was first mixed with the hardener, 4,4′-diaminophenyl sulfone (49 parts per 100 parts epoxy) and, after impregnation, was cured following the manufacturer's recommended protocol. As shown in Table 4, using cross-linked buckypaper as a starting point for impregnation resulted in substantially improved mechanical properties compared to direct impregnation of P-SWCNT buckypaper. This conclusion can also be drawn from other recent work demonstrating that buckypaper produced from epoxidized CNTs results in stronger composites after epoxy-impregnation than when impregnation was performed on unmodified CNT buckypaper,50 and suggests cross-linking is a promising route for composite fabrication.| Buckypaper | E (GPa) | UTS (MPa) |
|---|---|---|
| P-SWCNT | 0.37 ± 0.09 | 3.4 ± 0.9 |
| Epoxy-cross-linked (Int. B) | 2.1 ± 0.2 | 15 ± 2 |
| P-SWCNT, epoxy-impregnated | 4.3 ± 1.2 | 23 ± 10 |
| Epoxy-cross-linked (Int. B) + epoxy-impregnated | 7.5 ± 1.9 | 41 ± 8 |
Conclusions
3D covalently cross-linked SWCNT buckypapers were produced by wetting the initial buckypapers with external linkers and initiating chemical cross-linking under compression at elevated temperatures. SWCNT-OH buckypapers provided a common starting point for several cross-linking treatments. The properties of the resulting 3D covalently cross-linked buckypapers can be tailored through the selection of the external linkers and it is possible to improve strength, stiffness, and thermal conductivity, as well as maintain electrical conductivity through selection of the linking reagent. 3D covalently cross-linked buckypapers offer a route to improve mechanical properties while retaining the topology and porosity of the structure. The use of an epoxy-crosslinked buckypaper was also demonstrated to be an advantageous starting point for preparation of buckypaper composites by epoxy impregnation, where the combination of an epoxy cross-linking reagent followed by epoxy-impregnation provided superior mechanical properties in comparison to impregnation alone.Acknowledgements
The work at NRC was financially supported by CRTI-07-121RD. The authors acknowledge technical assistance from Gordon Chan and Malgosia Daroszewska for SEM and TGA data, respectively, and thank Dr Greg Lopinski for access to equipment for van der Pauw measurements. Thermal conductivity measurements were supported by NSERC and the Canada Foundation for Innovation, Atlantic Innovation Fund and other partners that fund the Facilities for Materials Characterization managed by the Institute for Research in Materials at Dalhousie University.Notes and references
- J. Liu, A. G. Rinzler, H. Dia, J. H. Hafner, R. K. Bradley, P. J. Boul, A. Lu, T. Iverson, K. Shelimov, C. B. Huffman, F. Rodriguez-Marcias, Y.-S. Shon, T. R. Lee, D. T. Colbert and R. E. Smalley, Fullerene Pipes, Science, 1998, 280, 1253–1256 CrossRef CAS.
- J. N. Coleman, W. J. Blau, A. B. Dalton, E. Munoz, S. Collins, B. G. Kim, J. Razal, M. Selvidge, G. Vieiro and R. H. Baughman, Appl. Phys. Lett., 2003, 82, 1682–1684 CrossRef CAS PubMed.
- L. Song, H. Zhang, Z. Zhang and S. Xie, Composites, Part A, 2007, 38, 388–392 CrossRef PubMed.
- Z. Wang, Z. Liang, B. Wang, C. Zhang and L. Kramer, Composites, Part A, 2004, 35, 1225–1232 CrossRef PubMed.
- S. Wang, Z. Liang, G. Pham, Y. B. Park, B. Wang, C. Zhang, L. Kramer and P. Funchess, Nanotechnology, 2007, 18, 095708 CrossRef.
- G. T. Pham, Y.-B. Park, S. Wang, Z. Liang, B. Wang, C. Zhang, P. Funchess and L. Kramer, Nanotechnology, 2008, 19, 325705 CrossRef PubMed.
- B. Ashrafi, J. Guan, V. Mirjalili, P. Hubert, B. Simard and A. Johnston, Composites, Part A, 2010, 41, 1184–1191 CrossRef PubMed.
- S. U. Khan and J.-K. Kim, Carbon, 2012, 50, 5265–5277 CrossRef CAS PubMed.
- X. Fu, C. Zhang, T. Liu, R. Liang and B. Wang, Nanotechnology, 2010, 21, 235701 CrossRef PubMed.
- J. G. Park, J. Louis, Q. Cheng, J. Bao, J. Smithyman, R. Liang, B. Wang, C. Zhang, J. S. Brooks, L. Kramer, P. Fanchasis and D. Dorough, Nanotechnology, 2009, 20, 415702 CrossRef PubMed.
- H. Chen, M. Chen, J. Di, G. Xu, H. Li and Q. Li, J. Phys. Chem. C, 2012, 116, 3903–3909 CAS.
- M. O. Memon and K. Lafdi, Carbon, 2011, 49, 3820–3828 CrossRef CAS PubMed.
- P.-J. Cottinet, M.-Q. Le, J. Degraff, C. Souders, Z. Liang, B. Wang and C. Zhang, Sens. Actuators, A, 2013, 194, 252–258 CrossRef CAS PubMed.
- P.-J. Cottinet, C. Souders, S.-Y. Tsai, R. Liang, B. Wang and C. Zhang, Phys. Lett. A, 2012, 376, 1132–1136 CrossRef CAS PubMed.
- P. Dharap, Z. Li, S. Nagarajaiah and E. V. Barrera, Nanotechnology, 2004, 15, 379–382 CrossRef CAS.
- S. M. Cooper, H. F. Chuang, M. Cinke, B. A. Cruden and M. Meyyappan, Nano Lett., 2003, 3, 189–192 CrossRef CAS.
- W. Knapp and D. Schleussner, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct., 2003, 21, 557–561 CrossRef CAS.
- S. Sfaelou, M. Antoniadou, G. Trakakis, V. Dracopoulos, D. Tasis, J. Parthenios, C. Galiotis, K. Papagelis and P. Lianos, Electrochim. Acta, 2012, 80, 399–404 CrossRef CAS PubMed.
- C. Zheng, W. Qian, Y. Yu and F. Wei, Particuology, 2013, 11, 409–414 CrossRef CAS PubMed.
- J. Che, P. Chen and M. B. Chan-Park, J. Mater. Chem. A, 2013, 1, 4057–4066 CAS.
- Y. Hu, X. Li, J. Wang, R. Li and X. Sun, J. Power Sources, 2013, 237, 41–46 CrossRef CAS PubMed.
- K. Evanoff, J. Benson, M. Schauer, I. Kovalenko, D. Lashmore, W. J. Ready and G. Yushin, ACS Nano, 2012, 6, 9837–9845 CrossRef CAS PubMed.
- N. Behabtu, M. J. Green and M. Pasquali, Nano Today, 2008, 3(5–6), 24–34 CrossRef CAS.
- B. I. Yacobson, G. Samsonidze and G. G. Samsonidze, Carbon, 2000, 38, 1675–1680 CrossRef.
- X. Zhang, T. V. Sreekumar, T. Liu and S. Kumar, J. Phys. Chem. B, 2004, 108, 16435–16440 CrossRef CAS.
- L. Berhan, Y. B. Yi, A. M. Sastry, E. Munoz, M. Selvidge and R. Baughman, J. Appl. Phys., 2004, 95, 4335–4345 CrossRef CAS PubMed.
- C. J. Frizzell, M. In Het Panhuis, D. H. Coutinho, K. J. Balkus, A. I. Minett, W. J. Blau and J. N. Coleman, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 1–8 CrossRef.
- K. Kobashi, T. Hirabayashi, S. Ata, T. Yamada, D. N. Futaba and K. Hata, Green, Scalable, ACS Appl. Mater. Interfaces, 2013, 5, 12602–12608 CAS.
- Q. Cheng, J. Bao, J. Park, Z. Liang, C. Zhang and B. Wang, Adv. Funct. Mater., 2009, 19, 3219–3225 CrossRef CAS.
- M. Terrones, H. Terrones, F. Banhart, J.-C. Charlier and P. M. Ajayan, Science, 2000, 288, 1226–1229 CrossRef CAS.
- B. Ni, R. Andrews, D. Jacques, D. Qian, M. B. J. Wijesundara, Y. Choi, L. Hanley and S. B. Sinnott, J. Phys. Chem. B, 2001, 105, 12719–12725 CrossRef CAS.
- E. Salonen, A. V. Krasheninnikov and K. Nordlund, Nucl. Instrum. Methods Phys. Res., Sect. B, 2002, 193, 603–608 CrossRef CAS.
- A. Kis, G. Csányi, J.-P. Salvetat, T.-N. Lee, E. Couteau, A. J. Kulik, W. Benoit, J. Brugger and L. Forró, Nat. Mater., 2004, 3, 153–157 CrossRef CAS PubMed.
- R. L. Federizzi, C. S. Moura and L. Amaral, J. Phys. Chem. B, 2006, 110, 23215–23220 CrossRef CAS PubMed.
- S. G. Miller, T. S. Williams, J. S. Baker, F. Solà, M. Lebron-Colon, L. S. McCorkle, N. G. Wilmoth, J. Gaier, M. Chen and M. A. Meador, ACS Appl. Mater. Interfaces, 2014, 6, 6120–6126 CAS.
- S. Steiner, S. Busato and P. Ermanni, Carbon, 2012, 50, 1713–1719 CrossRef CAS PubMed.
- J. Zhang, D. Jiang, H.-X. Peng and F. Qin, Carbon, 2013, 63, 125–132 CrossRef CAS PubMed.
- D. N. Ventura, R. A. Stone, K.-S. Chen, H. H. Hariri, K. A. Riddle, T. J. Fellers, C. S. Yun, G. F. Strouse, H. W. Kroto and S. F. A. Acquah, Carbon, 2010, 48, 987–994 CrossRef CAS PubMed.
- C. T. Kingston, Z. J. Jakubek, S. Dénommée and B. Simard, Carbon, 2004, 42, 1657–1664 CrossRef CAS PubMed.
- M. B. Jakubinek, M. B. Johnson, M. A. White, J. Guan and B. Simard, J. Nanosci. Nanotechnol., 2010, 10, 8151–8157 CrossRef CAS PubMed.
- E. Najafi, J. Wang, A. P. Hitchcock, J. W. Guan, S. Denommee and B. Simard, J. Am. Chem. Soc., 2010, 132, 9020–9029 CrossRef CAS PubMed.
- N. Tagmatarchis, V. Georgaklas, M. Prato and H. Shinohara, Chem. Commun., 2002, 2010–2011 RSC.
- J. H. Choi, S. B. Oh, J. Chang, I. Kim, C.-S. Ha, B. G. Kim, J. H. Han, S.-W. Joo, G.-W. Kim and H.-J. Paik, Polym. Bull., 2005, 55, 173–179 CrossRef CAS.
- J. W. Guan, R. Anderson and B. Simard, US Provisional 61–353737, PCT/CA2011/000683, June 10, 2010.
- B. M. Zawilski, R. T. Littleton and T. M. Tritt, Rev. Sci. Instrum., 2001, 72, 1770–1774 CrossRef CAS PubMed.
- M. B. Jakubinek, M. B. Johnson, M. A. White, C. Jayasinghe, G. Li, W. Cho, M. J. Schulz and V. Shanov, Carbon, 2012, 50, 244–248 CrossRef CAS PubMed.
- J. Pöhls, M. B. Johnson, M. A. White, R. Malik, B. Ruff, C. Jayasinghe, M. J. Schulz and V. Shanov, Carbon, 2012, 50, 4175–4183 CrossRef PubMed.
- C. T. Kingston, Y. Martinez-Rubi, J. Guan, M. Barnes, C. Scriver, R. E. Sturgeon and B. Simard, Anal. Bioanal. Chem., 2010, 396, 1037–1044 CrossRef CAS PubMed.
- Y. Martinez-Rubi, J. M. Gonzalez-Dominguez, A. Anson-Casaos, C. T. Kingston, M. Daroszewska, M. Barnes, P. Hubert, C. Cattin, M. T. Martinez and B. Simard, Nanotechnology, 2012, 23, 285701 CrossRef CAS PubMed.
- G. Trakakis, D. Tasis, C. Aggelopoulos, J. Parthenios, C. Galiotis and K. Papegelis, Compos. Sci. Technol., 2013, 77, 52–59 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Thermogravimetric analysis (TGA) data for 3D crosslinked buckypapers derived from SWCNT-OH buckypaper. See DOI: 10.1039/c4ra12026d |
| This journal is © The Royal Society of Chemistry 2014 |