Aqueous formic acid: an efficient, inexpensive and environmentally friendly organocatalyst for three-component Strecker synthesis of α-aminonitriles and imines with excellent yields
Abstract
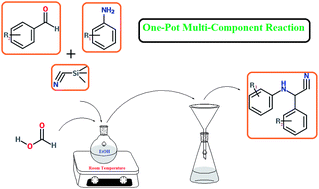
Aqueous formic acid (37%) which is an efficient, inexpensive and environmentally friendly organocatalyst was used in the Strecker reaction to afford α-aminonitriles and imines. Reaction was carried out under mild conditions and room temperature in high yields. We obtained α-aminonitrile derives by using trimethylsilyl cyanide through the Strecker reaction. The most important feature of formic acid as a catalyst is affordability and availability.

 Please wait while we load your content...
Please wait while we load your content...