Binding and removal of octahedral, tetrahedral, square planar and linear anions in water by means of activated carbon functionalized with a pyrimidine-based anion receptor†
Matteo Savastanoa,
Paloma Arranz-Mascarósb,
Carla Bazzicalupia,
Antonio Bianchi*a,
Claudia Giorgia,
M. Luz Godino-Salidob,
Maria Dolores Gutiérrez-Valerob and
Rafael López-Garzón*b
aDepartment of Chemistry “Ugo Schiff”, University of Florence, Via della Lastruccia 3, 50019, Sesto Fiorentino, Italy. E-mail: antonio.bianchi@unifi.it
bDepartment of Inorganic and Organic Chemistry, University of Jaén 23071, Jaén, Spain. E-mail: rlopez@ujaen.es
First published on 30th October 2014
Abstract
Binding of S2O32−, SeO42−, Pt(CN)42−, Co(CN)63−, Au(S2O3)23− and Fe(CN)64− anions by the protonated (positively charged) forms of tren (tris(2-aminoethyl)amine) and of the tren-derivative (HL) containing a pyrimidine residue was studied by means of potentiometric measurements in 0.1 M NMe4Cl solutions at 298.1 ± 0.1 K. Both ligands form stable complexes with these anions which appear to be mostly stabilized by electrostatic forces. In the case of HL, an anion–π interaction with the pyrimidine residue of the ligand also affords a significant contribution to complex stability. Some shape preference for tetrahedral and octahedral anions over square planar ones is observed. A hybrid AC/HL material obtained by adsorption of HL on commercial activated carbon (AC) was used to study the extraction of these anions from water. AC/HL shows enhanced adsorption capacity toward all the anions studied with respect to AC. This behavior is ascribed to the stronger interaction of anions with the HL function of AC/HL than with the Cπ-H3O+ sites of unfunctionalized AC. Of special interest is the enhancement of the adsorption capacities found for Au(S2O3)23− and Pt(CN)42−, two anions of great relevance for the extraction of platinum and gold from ores and from metallic wastes.
Introduction
Anion coordination chemistry, the binding of negatively charged species via non covalent interactions with Lewis acid receptors, is a dynamic research area attracting growing interest as a fertile source of applications.1–10 We have recently shown, for instance, that functionalization of an activated carbon (AC) with the polyamine molecule HL gives rise to a hybrid material (AC/HL) with marked affinity for various anions, thus providing a promising tool for anion sequestration and the remediation of contaminated media.11The method used for the functionalization of a commercially available AC was based on the irreversible π-stacking interaction between the pyrimidine anchor of HL and the arene centres of the graphite domains of AC (Fig. 1).11 The characteristics of the AC/HL material mostly relies on the properties of the functionalities implanted on the carbon surface that are transferred to the hybrid material.13 HL contains a tren unit (L1) (Chart 1), whose protonated forms are efficient anion receptors,14 and coherently also protonated forms of HL give rise to stable complexes with various anions, both inorganic and organic.11,15–17 With the latter ligand, the participation of anion–π interactions, involving the pyrimidine residue, can furnish further contributions to complex stability in addition to charge–charge attraction and hydrogen bonding. Indeed, crystal structures of HL complexes with anions such as HgCl42−, HgBr42−, CdI42− and Co(CN)63− showed very short (among the shortest ever reported) anion–π interactions.11,17,18 Accordingly, the AC/HL material is an efficient sorbent for anions such as SO42−, PO43−, AsO43−, CrO42− and HgCl42− in water over rather wide pH ranges.11 More recently, we showed that HL forms stable to very stable complexes with other tetrahedral anions (S2O32−, SeO42−) and with anions of different geometries such as P2O74−, P3O105− and Co(CN)63−.16,17
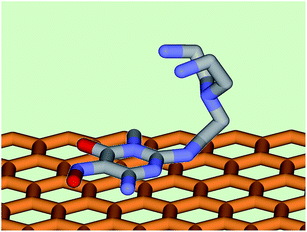 | ||
| Fig. 1 Schematic representation of the interaction between the surface of activated carbon and the pyrimidine residue of HL. | ||
 | ||
| Chart 1 Tren (L1) and tren derivative (HL) studied in this work. The notation HL is used for the pyrimidine derivative since it can undergo deprotonation of the NH group linked to the pyrimidine ring.12 | ||
In the present paper, we extend the study to metal complex anions such as Pt(CN)42−, Fe(CN)64− and Au(S2O3)23− and we relate the stability of S2O32−, SeO42−, Pt(CN)42−, Co(CN)63−, Fe(CN)64− and Au(S2O3)23− complexes of HL with the stability of the analogous species formed by the parent ligand tren. Furthermore, we analyse the sorption ability of the hybrid AC/HL material towards S2O32−, SeO42−, Pt(CN)42−, Co(CN)63−, Fe(CN)64− and Au(S2O3)23− to enlarge the base of data correlating the sorption properties of AC/HL with the binding ability of HL and of its constituents. Of particular interest was the study involving Pt(CN)42− and Au(S2O3)23−, since a successful adsorption of these anions on the hybrid AC/HL material could have important technological applications. Cyanide leaching of platinum-group metals (PGM) and gold followed by activated carbon adsorption of the resulting cyanide complexes is a technique currently in use, in particular for gold, and receives continuous attention for improvements and extensions for the recovery of these precious metals from ores or from metallic wastes such as automobile exhaust catalysts.19–22 Improvements of the activated carbon adsorption method is, of course, desirable, as well as replacement of the very toxic cyanide with safer ligands is desirable. Thiosulfate could be an alternative, at least in the case of gold.23 The very stable Au(S2O3)23− complex, for instance, is the main component obtained when thiosulfate is used as an alternative to cyanide for extraction of gold. Thiosulfate is essentially non-toxic and it is able to extract gold from ore types that are refractory to gold cyanidation, like carbonaceous or Carlin type ores. This alternative process, however, presents some drawbacks, the principal one being the lack of a suitable recovery technique, since Au(S2O3)23− is not efficiently adsorbed by activated carbon, which is the standard technique used in gold cyanidation to separate the gold complex from the ore slurry. As we will show later on in this paper, functionalization with HL enhances the ability of activated carbon to adsorb both Au(S2O3)23− and Pt(CN)42−.
Experimental
Materials
The HL ligand18 and the hybrid AC/HL material11,24 were prepared and characterized as previously described. The commercial AC adsorbent supplied by Merck was also previously characterized.23 The anions used for the potentiometric and the adsorption measurements were obtained as high purity Na2S2O3, Na2SeO4, Na3[Au(S2O3)2], K2[Pt(CN)4], K3[Co(CN)6] and K4[Fe(CN)6] salts from commercial sources and were used without further purification.Potentiometric measurements
pH-metric measurements (pH = −log[H+]) employed for the determination of equilibrium constants were carried out in 0.1 M NMe4Cl solutions at 298.1 ± 0.1 K, by using the equipment and the methodology that has been already described.25 The combined Hamilton glass electrode (LIQ-GLASS 238000/08) was calibrated as a hydrogen concentration probe by titrating known amounts of HCl with CO2-free NMe4OH solutions and determining the equivalent point by Gran's method26 which allows one to determine the standard potential E0 and the ionic product of water (pKw = 13.83(1) at 298.1 ± 0.1 K in 0.1 M NMe4Cl). At least three measurements were performed for each system in the pH ranges 2.5–10.5. The computer program Hyperquad27 was used to calculate the equilibrium constants from e.m.f. data. The concentration of the ligand was 1 × 10−3 M, while the concentration of anions [A] was in the range [L] ≤ [A] ≤ 5[L]. Ligand protonation constants used in calculations were previously determined,11,14 while anion protonation constants were taken from the literature.28 Three measurements were performed for each anion and the titration curves were treated both separately or as a unique set without significant variations in the calculated stability constants. The final stability constants were obtained by processing the whole set of curves.Adsorption measurements
Equilibration times for anion adsorption measurements were preliminarily determined by means of independent experiments. For this purpose, different flasks containing 25 mL of 10−3 M anion solution and 25 mg of adsorbent were prepared, kept under stirring, and the anion concentration was measured at different times by means of ICP mass measurements in all cases except for Pt(CN)42− whose concentration was measured by UV absorbance at 255 nm.The anion adsorption isotherms were obtained at 298.1 ± 0.1 K. Typically, 25 mg of adsorbent (AC or AC/HL) was added to a 100 mL plastic flask containing 25 mL of aqueous solution of the examined anion. Anion concentration was varied between 5 × 10−4 and 1.1 × 10−3 M, and the initial pH was adjusted to 6.0 by adding KOH or HCl solutions to the adsorbate solutions. The flasks were kept under stirring into a Selecta Unitronic-Orbital themostated air-bath and the anion concentration in the equilibrium solutions was determined as indicated above. UV measurements were previously performed to ascertain that no desorption of HL occurred in the presence of the studied anions under the experimental conditions employed, and blank experiments were performed to verify that neither the ligand nor the anions were adsorbed by the plastic flasks.
To obtain the theoretical maximum adsorption capacities of the corresponding sorbents toward the anions (Xm), the isotherm data X (mmols of anion adsorbed per gram of adsorbent) and Ceq (anion equilibrium concentrations) were fit to the linear form of the Langmuir equation29 1/X = 1/B × Xm × Ceq + 1/Xm, where B is the Langmuir constant. This was done for all the adsorbent/adsorbate systems (R varying between 0.9661 and 0.9915) except for S2O32− due to high dispersion of the experimental data obtained in this case.
XPS spectra of AC/Pt(CN)42− and AC/Au(S2O3)23− samples, obtained from the adsorption experiments of Pt(CN)42− and Au(S2O3)23− on AC, were registered with a ESCA5701 instrument (Physical Electronics), by using the Mgkα 300 W 15 kV radiation of twin anode in the constant analyzer energy mode, with pass energy of 187.85 eV (for the survey spectrum) and 29.35 eV (for narrow atomic ranges). Pressure of the analysis chamber was maintained at 4 × 10−9 Torr. The binding energy and the Auger kinetic energy scale were regulated by setting the C1s transition at 284.6 eV. The accuracy of BE values was ±0.2 eV.
Results and discussion
Anion complexation by HL and L1
The equilibrium constants for the interaction of S2O32−, SeO42−, Pt(CN)42−, Co(CN)63−, Fe(CN)64− and Au(S2O3)23− with protonated forms of the pyrimidine derivative HL and the parent ligand tren (L1) were determined by means of computer analysis, performed by means of the Hyperquad27 program, of potentiometric (pH-metric) titration data acquired in 0.1 M Me4NCl aqueous solutions at 298.1 K. Their values are listed in Tables 1 and 2, respectively.| Equilibria | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
|---|---|
| a Values in parentheses are standard deviations on the last significant figures.b Taken from ref. 17.c Determined by isothermal titration calorimetry. | |
| HL + S2O32− = [HL(S2O3)]2− | 1.9(1)a,b,c |
| H2L+ + S2O32− = [H2L(S2O3)]− | 2.83(7)b |
| H3L2+ + S2O32− = [H3L(S2O3)] | 3.34(8)b |
| H4L3+ + S2O32− = [H4L(S2O3)]+ | 3.95(9)b |
| HL + SeO42− = [HL(SeO4)]2− | 2.1(1)b,c |
| H2L+ + SeO42− = [H2L(SeO4)]− | 2.68(6)b |
| H3L2+ + SeO42− = [H3L(SeO4)] | 3.34(6)b |
| H4L3+ + SeO42− = [H4L(SeO4)]+ | 3.96(8)b |
| H2L+ + Pt(CN)42− = [H2L(Pt(CN)4)]− | 2.24(6)b |
| H3L2+ + Pt(CN)42− = [H3L(Pt(CN)4)] | 2.62(6)b |
| H4L3+ + Pt(CN)42− = [H4L(Pt(CN)4)]+ | 2.74(5)b |
| HL + Co(CN)63− = [HL(Co(CN)6)]3− | 2.0(1)b,c |
| H2L+ + Co(CN)63− = [H2L(Co(CN)6)]2− | 2.72(6)b |
| H3L2+ + Co(CN)63− = [H3L(Co(CN)6)]− | 3.44(5)b |
| H4L3+ + Co(CN)63− = [H4L(Co(CN)6)] | 4.24(6)b |
| H2L+ + Fe(CN)64− = [H2L(Fe(CN)6)]3− | 2.91(8) |
| H3L2+ + Fe(CN)64− = [H3L(Fe(CN)6)]2− | 3.65(6) |
| H4L3+ + Fe(CN)64− = [H4L(Fe(CN)6)]− | 5.08(9) |
| H4L3+ + HFe(CN)63− = [H4L(HFe(CN)6)] | 4.21(8) |
| H2L+ + Au(S2O3)23− = [H2L(Au(S2O3)2)]2− | 2.33(7) |
| H3L2+ + Au(S2O3)23− = [H3L(Au(S2O3)2)]− | 3.31(5) |
| H4L3+ + Au(S2O3)23− = [H4L(Au(S2O3)2)] | 3.86(6) |
| Equilibria | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
|---|---|
| a Values in parentheses are standard deviations on the last significant figures. | |
| H2L12+ + S2O32− = [H2L1(S2O3)] | 1.61(8)a |
| H3L13+ + S2O32− = [H3L1(S2O3)]+ | 2.08(7) |
| H4L14+ + S2O32− = [H4L1(S2O3)]2+ | 2.97(7) |
| H2L12+ + SeO42− = [H2L1(SeO4)] | 1.58(6) |
| H3L13+ + SeO42− = [H3L1(SeO4)]+ | 1.93(6) |
| H4L14+ + SeO42− = [H4L1(SeO4)]2+ | 3.28(4) |
| HL1+ + Pt(CN)42− = [HL1(Pt(CN)4)]− | 2.03(8) |
| H2L12+ + Pt(CN)42− = [H2L1(Pt(CN)4)] | 2.26(7) |
| H3L13+ + Pt(CN)42− = [H3L1(Pt(CN)4)]+ | 2.18(7) |
| HL1+ + Co(CN)63− = [HL1(Co(CN)6)]2− | 3.11(9) |
| H2L12+ + Co(CN)63− = [H2L1(Co(CN)6)]− | 3.30(7) |
| H3L13+ + Co(CN)63− = [H3L1(Co(CN)6)] | 3.82(6) |
| H4L14+ + Co(CN)63− = [H4L1(Co(CN)6)]+ | 4.88(7) |
| HL1+ + Fe(CN)64− = [HL1(Fe(CN)6)]3− | 3.24(6) |
| H2L12+ + Fe(CN)64− = [H2L1(Fe(CN)6)]2− | 3.42(7) |
| H3L13+ + Fe(CN)64− = [H3L1(Fe(CN)6)]− | 3.99(8) |
| H4L14+ + Fe(CN)64− = [H4L1(Fe(CN)6)] | 5.47(6) |
| H2L12+ + Au(S2O3)23− = [H2L1(Au(S2O3)2)]− | 2.23(5) |
| H3L13+ + Au(S2O3)23− = [H3L1(Au(S2O3)2)] | 2.47(8) |
| H4L14+ + Au(S2O3)23− = [H4L1(Au(S2O3)2)]+ | 2.66(6) |
As can be seen from these tables, the stability constants of these complexes invariably increases as increasing positive charge accumulates on the ligands upon protonation. Only in the case of Pt(CN)42−, the effect of ligand charge on complex stability is small or unappreciable within the experimental errors.
The two ligands display rather similar anion binding trends. For a given ligand charge, for instance, the tetrahedral anions S2O32− and SeO42− show very similar binding constants, and similar behaviors were previously found for SO42−, HPO42− and HAsO42−.11,14 Also the stability of complexes with the elongated Au(S2O3)23− anion, having tetrahedral terminations, are comparable with the stability of complexes with the above tetrahedral anions, despite its greater charge. The more charged octahedral Co(CN)63− and Fe(CN)64− anions form complexes of greater stability, relative to the less charged anions, Fe(CN)64− being the anion forming the most stable complexes. On the other hand, complexes of the square planar Pt(CN)42− with HL are less stable than the analogous species formed by the other dicharged anions, and reduced stability is also observed for the complex with H3L13+.
Accordingly, anion binding by protonated forms of HL and L1 appears to be mostly regulated by electrostatic forces, and the ligands display some shape preference for tetrahedral and octahedral anions over square planar ones. Indeed, the crystal structures of the complexes formed by HgCl42−, HgBr42− and CdI42− with H3L2+ and by Co(CN)63− with H4L3+ showed a good complementarity between the interacting partners, the anions being anchored to the ligand via salt-bridges with ligand ammonium groups and very strong anion–π interactions with the pyrimidine residue of HL acting as anion binding functionality (Fig. 2).11,17,18 In particular, the H4L[Co(CN)6] complex showed one of the strongest anion–π interactions so far reported.17
 | ||
| Fig. 2 Crystal structures of the complexes formed by H4L3+ with Co(CN)63− (a)17 and by H3L2+ with HgBr42− (b) HgCl42− (c)11 and CdI42− (d)18 (CSD refcodes IDIKAJ (a), AVISEE (b), AVISII (c), WASXAQ (d)). | ||
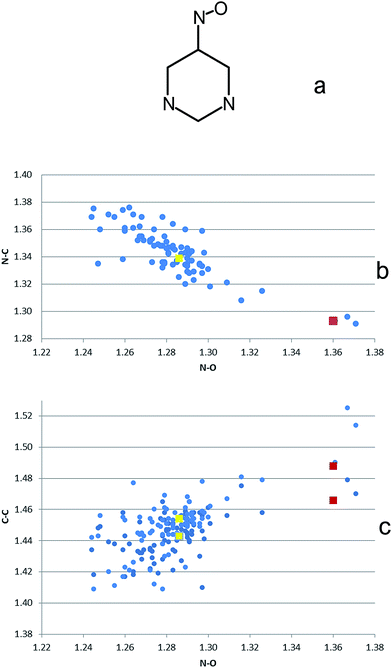
While in H3L2+ protonation involves the two primary amine groups of the tren moiety, in H4L3+ also the pyrimidine nitroso group is protonated. Since protonation of the nitroso group modifies its π-electron cloud, we expect that it also produces a significant alteration of the π-density on the pyrimidine ring, thus affecting the anion–π interactions occurring in these complexes. To get insight on this point, we performed a detailed analysis of the crystal structures deposited in the Cambridge Structural Database (CSD) for nitroso-pyrimidines containing nitroso groups in both neutral and protonated forms, by using the structure reported in Fig. 3a as a query for the database investigation and excluding metal coordinated NO groups. No limits to data quality were imposed. Visualization of these crystal structures evidences that protonation of the nitroso group determines, in addition to the expected lengthening of the N–O bonds and shortening of the adjacent N–C bonds, a significant elongation of the C–C distances of the pyrimidine ring. This is clearly shown in Fig. 3 by the correlations of N–C (Fig. 3b) and C–C (Fig. 3c) with N–O bond distances obtained from CSD. In this figure, data relative to the {H4L[Co(CN)6]}·2H2O complex17 are displayed in red, while the yellow markers represent the mean value evaluated for the structures of complexes formed by H3L2+ with HgCl42−, HgBr42− and CdI42−.11,18 Accordingly, protonation of the nitroso group has an electron withdrawing effect on the pyrimidine π-electron density: the N–O bond acquires single bond character, the bond between the nitroso nitrogen and the linked carbon atom is shortened to a double bond distance, while the loss of π-electron density of the pyrimidine ring is particularly evident for the C–C bonds close to the nitroso group.
To verify whether the change in π-electron density brought about by protonation of the nitroso group affects the anion–π interaction with our ligand in the solid state, we overlaid the nitroso-pyrimidine group in the crystal structures of the anion complexes with H3L2+ and H4L3+ and placed the anion atoms forming anion–π interactions in their relative positions to obtain the picture shown in Fig. 4. It is clearly visible that the interacting CN− group in the {H4L[Co(CN)6]}·2H2O complex is more displaced from the center of the pyrimidine ring than any other anion, and that it points towards the C–C bonds where a lower π-electron density is localized.
Evidences that also in aqueous solution anion–π interactions contribute to stabilize these anion complexes were obtained by means of isothermal titration measurements and by linear correlation of complex stability constants with ligand charge, which provided evaluations of the equilibrium constants for SO42−, S2O32−, SeO42−, and Co(CN)63− association with the uncharged HL ligand to be in the range log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 1.6–2.2.11,17 Values for S2O32−, SeO42−, and Co(CN)63− complexes with HL are shown in Table 1.
K = 1.6–2.2.11,17 Values for S2O32−, SeO42−, and Co(CN)63− complexes with HL are shown in Table 1.
As far as the anion binding abilities of the two ligands are compared, we observe that, for ligand species with the same positive charge, HL forms more stable complexes than L1, the complexes of Co(CN)63− and Fe(CN)64− with the monocharged H2L+ and HL1+ receptors representing the unique exceptions to this trend (Tables 1 and 2). Most likely, the anion–π interactions and the less solvated environment provided by the pyrimidine residue of HL make the main contributions that enhance the ligand binding ability. Nevertheless, an interpretation of ligand binding ability in terms of binding selectivity cannot be performed by simply comparing the stability constants of analogous complexes, since the formation of these complexes is regulated by the protonation of ligands having different protonation properties. A useful method for the analysis of binding selectivity consists in considering a ternary system containing the anion and the two ligands, in equimolar amounts, and calculating the overall percentage of complexes formed by each ligand at different pH values.30,31 The result of a similar analysis performed for the system HL/L1/SeO42− (millimolar concentrations) is reported in the diagram of Fig. 5a, showing that the anion is preferentially bound by HL over the entire pH range in which complexation occurs. Conversely, the same analysis performed for the HL/L1/Fe(CN)64− system reveals that L1 is the preferred ligand by Fe(CN)64− (Fig. 5b). The results obtained for the other anions are displayed in Fig. S1 of ESI.† While the hexacyanometallate anions undergo preferential binding with HL, all the other anions prefer L1. Analogous results (see Fig. S2†) are obtained by comparison of the conditional stability constants calculated for the complexation equilibria A(all forms) + L(all forms) = AB(all forms) and expressed as KcondAB = [AL(all forms)]/([A(all forms)][L(all forms)]), where A, L and AL are the anion, the ligand and the anion complex, respectively.31
In conclusion, the ligand tren (L1) maintains its anion binding properties upon functionalization to give its pyrimidine derivative HL. In some cases (S2O32−, SeO42−, Pt(CN)42−, Au(S2O3)23−), HL displays enhanced binding ability relative to tren, while in few case (Co(CN)63−, Fe(CN)64−) it is less efficient than the parent ligand. Nevertheless, in all cases HL is a promising candidate for the preparation of hybrid AC/HL materials for recovery of these anions from aqueous media.
Adsorption studies
The use of AC-based adsorbents is important compared to other sorbents because of its large surface area, high adsorption capacity, porous structure, negligible environmental toxicity, low cost, and high purity.21 In previous works it was established that the hybrid AC/HL material behaves as an efficient adsorbent for SO42−, PO43−, AsO43−, HgCl42− and CrO42− anions11 as well as for some metal cations.18,24,32 Therefore, having in mind the binding data shown in the previous section, it seemed interesting to extend the adsorption study to S2O32−, SeO42−, Pt(CN)42−, Co(CN)63−, Au(S2O3)23− and Fe(CN)64− anions, especially to Pt(CN)42− and Au(S2O3)23− because of the role they have, or might have, in precious metals extraction. To this purpose, we analyzed the adsorptivity toward the above set of anions of a commercial activated carbon (AC) and of the corresponding AC/HL hybrid material that was prepared as previously described.11,24AC is a graphitized activated carbon with a surface area of 1062 m2 g−1 and containing oxygen as the only heteroatom component present in significant amount (3.9%).11,24 Most of the surface area (ca. 1000 m2 g−1) is associated to narrow micropores (diameter < 2.5–3.0 nm) whereas the remaining area corresponds to mesopores (2.5–3 nm < diameter > 50 nm) and macropores (diameter > 50 nm).33 AC surface mainly exhibits weak Brönsted basic character,11 which is reflected in its ability to bind protons from water giving rise to the accumulation of net positive charge in the 3.0–9.2 pH range. The low proton affinities of the (few) oxygen groups on the AC surface (carboxyl acids, carbonyl, quinone, lactone and phenol groups) exclude these functions from acting as binding sites for protons in the 2.5–10.0 pH range. Consequently, most of proton binding sites at AC surface are expected to be the arene centers of the graphite sheets (Cπ), which interact with protons according to the Cπ + H3O+ → Cπ-H3O+ process.11,34 The surface charge density, Q (mmol H+ per gram of AC), decreases steadily from ca. 0.26 mmol H+ per g, at pH 3.5, to 0.15 mmol of H+ per g at pH = 6.0.11
The textural and surface chemical properties of AC/HL have been described previously. The hybrid material AC/HL contains 0.49 mmol of HL per gram of AC, irreversibly adsorbed in water in the pH range 2.5–10. The specific surface area, pore distribution and surface chemical properties of AC/HL were also determined previously.11,33
Attachment of HL to the surface of AC, by π–π stacking interaction of the aromatic residue of HL with the Cπ sites AC,24 has two effects on the physico-chemical properties of the latter: (i) transference of the Brönsted base properties of the amine residue of HL to the AC surface, determining a sharp increase of the positive surface charge of AC/HL, relative to AC, in the whole pH range,11 and (ii) blocking of the entrance to the more inner pores of AC by HL molecules that reduces the accessible surface area of AC/HL to 495 m2 g−1.33
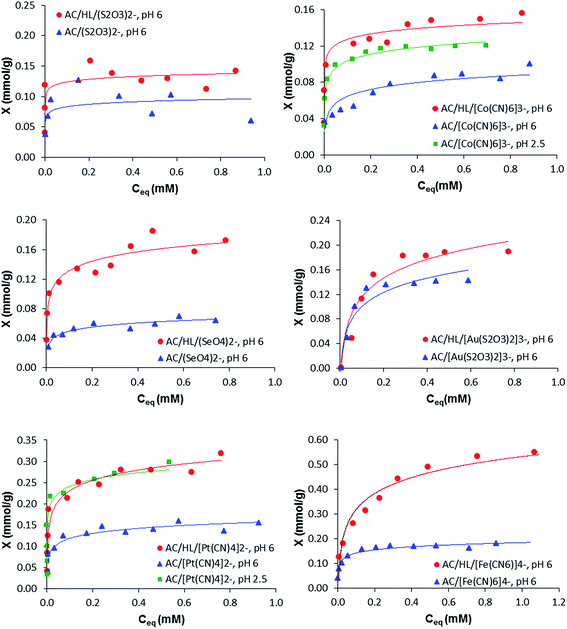
For the studies of anion adsorption, pH 6.0 was chosen as the most suitable for comparative purposes, since at this pH all these anions form the same unique H3LAn− (n = 0–2) complex (see Table 1). The adsorption isotherms on AC and AC/HL at pH 6.0 are shown in Fig. 6.
All the isotherms, except that of S2O32−, fit well to the Langmuir equation29 (see Experimental section), which enabled to obtain the maximum adsorption capacities, XmAC and XmAC/HL, summarized in Table 3, although some deviation of experimental points from the calculated curves, that can be ascribed to the surface heterogeneity of the porous adsorbents, is observed. As can be seen in Fig. 6, the adsorption capacity of AC grows, in most of cases, when the anion charge increases. Since the AC surface charge is the same for all systems, it is likely that anion adsorption on AC is due to electrostatic attraction with the positive charges of the protonated arene centers of AC, even if some dispersive contribution is also expected. The good fitting to Langmuir equation of the experimental isotherms (except for S2O32−) is consistent with the existence of similar adsorbing sites on the adsorbent surface. The lowest adsorptivities are found for di-charged anions (S2O32−, SeO42−), whereas intermediate adsorptivities correspond to the tri-charged Co(CN)63− and Au(S2O3)23− and the highest adsorptivity corresponds to the tetra-charged Fe(CN)64−. In the last case, not only the higher negative charge, but also the possibility hydrogen bonding with –OH functions (from phenol groups) should be responsible for the higher adsorptivity observed for this anion.
| Anion species | XmAC (mmol g−1) | XmAC/HL (mmol g−1) | Improvement factor | XNmAC (mmol m−2) | XNmAC/HL (mmol m−2) | Normalized improvement factor |
|---|---|---|---|---|---|---|
| a Approximate values from the corresponding isotherms (Fig. 6).b Linear correlation coefficients (R).c Values in parentheses are standard deviations on the last significant figure. | ||||||
| S2O32− | 0.08a | 0.12a | 2 | 4.85 × 10−5 | 2.02 × 10−4 | 4.16 |
| SeO42− | 0.060(2) [0.9690]b | 0.138(8) [0.9840]b | 2.30 | 5.7 × 10−5(2)c | 2.8 × 10−4(2) | 4.91 |
| Pt(CN)42− | 0.142(4) [0.9719]b | 0.30(1) [0.9662]b | 2.11 | 1.34 × 10−4(3) | 6.1 × 10−4(2) | 4.55 |
| Au(S2O3)23− | 0.157(6) [0.9664]b | 0.209(6) [0.9661]b | 1.33 | 1.48 × 10−4(5) | 4.2 × 10−4(1) | 2.84 |
| Co(CN)63− | 0.098(6) [0.9795]b | 0.161(3) [0.9798]b | 1.65 | 9.2 × 10−5(5) | 3.26 × 10−4(6) | 3.54 |
| Fe(CN)64− | 0.172(2) [0.9866]b | 0.54(2) [0.9915]b | 3.14 | 1.62 × 10−4(2) | 1.10 × 10−3(4) | 6.79 |
It is worth commenting the adsorption of Pt(CN)42− and Au(S2O3)23−. The adsorptivity on AC of the elongated tri-charged Au(S2O3)23− is higher than that of Co(CN)63−, while adsorptivity of planar Pt(CN)42− is greater than that of all anions but Fe(CN)64−. The absence of reduced Pt(0) and Au(0) in the XPS spectra of samples of AC/Pt(CN)42− and AC/Au(S2O3)23−, respectively, rules out metal reduction as the cause of the high adsorptivities of these anions on AC. Three effects could contribute to the higher adsorptivities of this couple of anions: (i) their high hydrophobicity due to their high molecular sizes that determine lower water solubilities;35 (ii) the existence of Cπ–dπ interactions between the arene centers of AC and the metal center of linear Au(S2O3)23− and planar Pt(CN)42− complexes,36 although for Au(S2O3)23− such interactions should be somewhat hindered by the non-planar structure of S2O32−,37 (iii) Cπ–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Nπ interactions between the arene centers of AC and the metal coordinated cyanide anions. Anyway, the influence of electrostatic components in anion interactions with the AC surface is clearly illustrated by the adsorptivity drop observed for Pt(CN)42− and Co(CN)63− from pH 2.5 to pH 6.0 (Fig. 6) as the positive net surface charge of AC decreases.
Nπ interactions between the arene centers of AC and the metal coordinated cyanide anions. Anyway, the influence of electrostatic components in anion interactions with the AC surface is clearly illustrated by the adsorptivity drop observed for Pt(CN)42− and Co(CN)63− from pH 2.5 to pH 6.0 (Fig. 6) as the positive net surface charge of AC decreases.
Also isotherms for anion adsorption at pH 6.0 on the hybrid AC/HL material (see Experimental section) are shown in Fig. 6, while the corresponding maximum adsorption capacities, XmAC/HL, are summarized in Table 3. These data evidence that functionalization of AC with HL clearly improves its adsorption capacity. Assuming that most of HL molecules are anchored to AC through the pyrimidine moiety,24,38 the enhanced adsorptivity of AC/HL can be ascribed to the high anion binding ability of the polyamine residue of HL.
In the cases of bi-anions S2O32−, SeO42−, Pt(CN)42−, the maximum adsorption capacities of AC/HL is about twice those of AC, which is consistent with the similar values of the stability constants of the H3LA neutral complexes formed by these anions. In the cases of Co(CN)63− and Au(S2O3)23−, we observed a lower enhancement. Although the stability constants of the H3LA complexes formed by these tri-anions are similar to those of the bi-anions, the monoanionic character of [H3L(Co(CN)6)]− and [H3L(Au(S2O3)2)]− determines higher water solubility, thus explaining their lower adsorptivities. Finally, the adsorptivity of AC/HL for Fe(CN)64− is enhanced by a factor of ca. 3, relative to AC, in agreement with the higher stability of the [H3L(Fe(CN)6)]2−complex formed at pH 6.0. Worth mentioning is the enhancement of the adsorption capacity observed for Au(S2O3)23− and Pt(CN)42− anions, since it may rise applicative interest for extraction and recovery of gold and platinum. The sequestering capacities of AC/HL toward these anions at pH 6.0 (39.4 and 58.7 mg g−1 of adsorbent, respectively) are comparable with or better than those obtained with other functionalized activated carbons.39,40
In summary, this study points out that functionalization of AC with the polyamine HL provides a hybrid material which significantly improves the ability of AC in the sequestration of the studied anions. This effect is a consequence of the anion binding ability of HL that is basically preserved after its adsorption onto the AC surface. These results enlarge a previous data base that correlates the metal ion and anion adsorbing properties of hybrid materials of AC/HL type with the ability of the HL ligand to bind the same species,11,18,24,32 and encourage further thorough research on the factors that could improve the adsorption capacity, i.e. (i) the influence of the textural characteristics of AC and (ii) the design of the polyamine HL function.
Regarding the textural properties, adsorption of HL on AC surface results in blocking the entrance to the more inner (narrower) pores, giving rise to a decrease of surface area from 1062 m2 g−1 for AC to 495 m2 g−1 for AC/HL,33 thus reducing the accessible surface area for larger functionalization with HL and interaction with other small molecular species (such as the anions here studied). Accordingly, a more suitable information on the improvement of adsorptivity derived from AC functionalization with HL is provided by the comparison of the adsorption capacities of both adsorbents normalized with respect to the accessible areas, XmN. The values of XmN for AC and AC/HL, that appear in Table 3, were obtained by dividing the maximum adsorption capacities, XmAC and XmAC/HL, by the accessible surface areas of the corresponding adsorbents. The improvement factors obtained by using such normalized values (see Table 3) increase greatly, thus showing that activated carbons having most of their surface areas accessible to the receptors (highly meso- and macroporous activated carbons) are the most suitable for ion recovery purposes.
With respect to the second point, the use of receptors containing a larger number of amine groups than HL should improve the anion binding ability of the hybrid material, since a greater positive charge would be achievable on the surface of the hybrid material. Furthermore, appropriate synthesis of tailored polyamine ligands, in particular of macrocyclic and cleft-like structures, would afford hybrid materials with enhanced anion extraction selectivity.
References
- (a) J. L. Sessler, P. A. Gale and W. S. Cho, Anion Receptor Chemistry, Royal Society of Chemistry, Cambridge, 2006 Search PubMed; (b) Supramolecular Chemistry of Anions, ed. A. Bianchi, K. Bowman-James and E. Garcia-España, Wiley-VCH, New York, 1997 Search PubMed; (c) J.-M. Lehn, Supramolecular Chemistry, Concepts and Perspectives, VCH, Weinheim, 1995 Search PubMed.
- H.-J. Schneider and A. K. Yatsimirsky, Chem. Soc. Rev., 2008, 37, 263 RSC.
- E. Garcia-España, P. Díaz, J. M. Llinares and A. Bianchi, Coord. Chem. Rev., 2006, 250, 2952 CrossRef.
- S. Kubik, C. Reyheller and S. Stüwe, J. Inclusion Phenom. Macrocyclic Chem., 2005, 52, 137 CrossRef CAS.
- (a) S. O. Kang, Md. A. Hossain and K. Bowman-James, Coord. Chem. Rev., 2006, 250, 3038 CrossRef CAS; (b) V. McKee, J. Nelson and R. M. Town, Chem. Soc. Rev., 2003, 32, 309 RSC.
- P. A. Gale, Coord. Chem. Rev., 2001, 213, 79 CrossRef CAS.
- P. D. Beer and E. J. Hayes, Coord. Chem. Rev., 2003, 240, 167 CrossRef CAS.
- R. Vilar, Angew. Chem., Int. Ed., 2003, 42, 1460 CrossRef CAS PubMed.
- J. M. Llinares, D. Powell and K. Bowman-James, Coord. Chem. Rev., 2003, 240, 57 CrossRef CAS.
- Special Issue on Anion SensingTop. Curr. Chem., 2005, 255 Search PubMed.
- P. Arranz, A. Bianchi, R. Cuesta, C. Giorgi, M. L. Godino, M. D. Gutiérrez, R. López and A. Santiago, Inorg. Chem., 2010, 49, 9321 CrossRef CAS PubMed.
- HL behaves as a week acid with pKa = 10.94 for the equilibrium HL = L− + H+ (see ref. 11).
- R. López-Garzón, M. L. Godino-Salido, M. D. Gutiérrez-Valero, P. Arranz-Mascaros, M. Melguizo, C. García, M. Domingo-García and F. J. López-Garzón, Inorg. Chim. Acta, 2014, 417, 208 CrossRef.
- C. Bazzicalupi, A. Bencini, A. Bianchi, A. Danesi, C. Giorgi and B. Valtancoli, Inorg. Chem., 2009, 48, 2391 CrossRef CAS PubMed.
- P. Arranz Mascaros, C. Bazzicalupi, A. Bianchi, C. Giorgi, M. D. Gutiérrez Valero, R. López Garzón, M. L. Godino Salido and B. Valtancoli, Chem. Commun., 2011, 47, 2814 RSC.
- P. Arranz Mascaros, C. Bazzicalupi, A. Bianchi, C. Giorgi, M. L. Godino Salido, M. D. Gutiérrez Valero, R. López Garzón and B. Valtancoli, New J. Chem., 2011, 35, 1883 RSC.
- P. Arranz Mascaros, C. Bazzicalupi, A. Bianchi, C. Giorgi, M. L. Godino Salido, M. D. Gutiérrez Valero, R. López Garzón and M. Savastano, J. Am. Chem. Soc., 2013, 135, 102 CrossRef CAS PubMed.
- J. García Martín, R. López Garzón, M. L. Godino Salido, R. Cuesta, M. D. Gutiérrez Valero, P. Arranz Mascaros and H. Stoeckli- Evans, Eur. J. Inorg. Chem., 2005, 3093 CrossRef.
- J. C. Yannopoulos, The Extractive Metallurgy of Gold, Van Nostrand Reinhold, New York, 1991 Search PubMed.
- M. G. Aylmore and D. M. Muir, Miner. Eng., 2001, 14, 135 CrossRef CAS.
- C. A. Snyders, C. N. Mpinga, S. M. Bradshaw, G. Akdogan and J. J. Eksteen, J. South. Afr. Inst. Min. Metall., 2013, 113, 381 CAS.
- C. Jing and H. Kun, Hydrometallurgy, 2006, 82, 164–171 CrossRef.
- H. Arima, T. Fujita and W.-T. Yen, Mater. Trans., 2003, 44, 2099 CrossRef CAS.
- J. García-Martín, R. López-Garzón, M. Godino-Salido, M. Gutiérrez-Valero, P. Arranz-Mascarós, R. Cuesta and F. Carrasco-Marín, Langmuir, 2005, 21, 6908 CrossRef PubMed.
- C. Bazzicalupi, A. Bianchi, C. Giorgi, P. Gratteri, P. Mariani and B. Valtancoli, Inorg. Chem., 2013, 52, 2125 CrossRef CAS PubMed.
- (a) G. Gran, Analyst, 1952, 77, 661 RSC; (b) F. J. Rossotti and H. Rossotti, J. Chem. Educ., 1965, 42, 375 CrossRef CAS.
- P. Gans, A. Sabatini and A. Vacca, Talanta, 1996, 43, 1739 CrossRef CAS PubMed.
- R. M. Smith and A. E. Martell, NIST Stability Constants Database, Version 4.0, National Institute of Standards and Technology, Washington, 1997 Search PubMed.
- M. D. Gutiérrez-Valero, P. Arranz-Mascarós, M. L. Godino-Salido, M. D. López-León, R. López-Garzón and R. Cuesta, Micropor. Mesopor. Mat., 2008, 116, 445 CrossRef.
- A. Bianchi and E. Garcia-España, J. Chem. Educ., 1999, 76, 1727 CrossRef CAS.
- C. Bazzicalupi, A. Bianchi, C. Giorgi, M. P. Clares and E. García-España, Coord. Chem. Rev., 2012, 256, 13 CrossRef CAS.
- M. L. Godino-Salido, R. López-Garzón, P. Arranz-Mascarós, M. D. Gutiérrez-Valero, A. Santiago-Medina and J. García-Martín, Polyhedron, 2009, 28, 3781 CrossRef CAS.
- M. D. Gutiérrez-Valero, P. Arranz-Mascarós, A. Peñas-Sanjuan, M. L. Godino-Salido, R. López-Garzón, A. Santiago-Medina, M. Melguizo-Guijarro, M. Pérez-Mendoza, F. J. López-Garzón and M. Domingo-García, Mater. Chem. Phys., 2012, 134, 608 CrossRef.
- L. R. Radovic, C. Moreno-Castilla and J. Rivera-Utrilla, Carbon materials as adsorbents in aqueous solution, in Chemistry and physics of carbon, ed. L. R. Radovic, Marcel Dekker, New York, 2000, vol. 27, pp. 227–405 Search PubMed.
- G. Wulfsberg, Inorganic Chemistry, University Science Book, Herndon, VA, 2000, ch. 2 Search PubMed.
- N. V. Vorob'ev-Desyatovskii, S. A. Kubyshkin, R. I. Ibragimova, V. V. Kaichev, Y. A. Dubrovskii, V. N. Babakov and D. A. Pichugina, Russ. J. Gen. Chem., 2012, 82, 384 CrossRef.
- R. F. Baggio and S. Baggio, J. Inorg. Nucl. Chem., 1973, 35, 3191 CrossRef CAS.
- M. D. Gutiérrez-Valero, M. L. Godino-Salido, P. Arranz-Mascarós, R. López-Garzón, R. Cuesta and J. García-Martín, Langmuir, 2007, 23, 5995 CrossRef PubMed.
- Z. Tu, S. Lu, X. Chang, Z. Li, Z. Hu, L. Zhang and H. Tian, Microchim. Acta, 2011, 173, 231 CrossRef CAS.
- H. Kasaini, F. Mahange and K. K. Mbaya, Proceedings of International Mineral Processing Congress, ed. W. D. Duo, Beijing, China, 24th edn, 24–28 Sept. 2008, vol. 2, pp. 3117–3123 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Selectivity diagrams and conditional stability constant diagrams for complex systems. Tables of overall equilibrium constants for complexation reactions. See DOI: 10.1039/c4ra11916a |
| This journal is © The Royal Society of Chemistry 2014 |