Enantioselective absorption of enantiomers with maleic anhydride-β-cyclodextrin modified magnetic microspheres†
Jun Huang,
Ping Su,
Jingwei Wu and
Yi Yang*
College of Science & Beijing Key Laboratory of Environmentally Harmful Chemical Analysis, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: yangyi@mail.buct.edu.cn; Fax: +86-10-64434898; Tel: +86-10-64454599
First published on 27th October 2014
Abstract
Multifunctional magnetic microspheres have enormous potential in diverse fields. In this work, surface chiral-modified magnetic microspheres as chiral selectors, are prepared by polymerizing maleic anhydride-β-cyclodextrin (MAH-β-CD) for the enantioselective absorption of four enantiomers. The successful grafting of MAH-β-CD onto the surface of magnetic microspheres is confirmed by transmission electron microscopy, X-ray diffraction, Fourier transform infrared spectroscopy and thermogravimetric analysis. The prepared functional microspheres have a three-ply structure with an average particle size of 550 nm and a high saturation magnetization of 60 emu g−1. The amount of MAH-β-CD modified on the P(MBAAm)@Fe3O4 was about 149.1 mg g−1. The analysis results of specific rotation and capillary electrophoresis reveal that the MAH-β-CD-modified Fe3O4 microspheres show stronger complexation of (−)-enantiomers than (+)-enantiomers. In addition, the MAH-β-CD-modified Fe3O4 microspheres have stronger enantioselective absorption for double-ring chiral compounds. These chiral-functionalized magnetic microspheres are therefore expected to be an efficient and economical chiral separation method for use in further research.
1. Introduction
The importance of chirality is undeniable in pharmaceutical, clinical, environment and food analysis, because many chiral compounds exhibit dramatically different bioactivity and pharmacological properties in stereo environments, sometimes with deleterious effects.1–4 Hence, the separation and analysis of chiral compounds is significantly important for the research of chiral compounds, spurring the development of a number of analytical methods, such as high performance liquid chromatography (HPLC),5 gas chromatography (GC),6 capillary electrophoresis (CE)7 and supercritical fluid chromatography.8 The chiral separations are achieved through the recognition between chiral selectors and enantiomeric compounds in the building stereo environments.9–11 Cyclodextrin (CD) derivatives, exhibiting a high chiral recognition to a number of chiral enantiomers, are the most common chiral selectors because of the host molecules with large ring-like structures, water solubility and affordability.12 Among the CD derivatives, polymerized CD usually provides a superior enantioseparation because of a different stereochemical conformation, the increasing number of the cavity, the influence of the chain length, as well as the degree of substitution.13 Hughes et al. prepared polymerized β-cyclodetrin (β-CD)-functionalized monoliths for the rapid separation of isoflavonoid puerarin and showed that polymeric β-CD markedly increased the β-CD ligand as well as the selectivity of the monoliths.14 Yang et al. reported a novel polymerized β-CD with an oligo (lactic acid) group, which was then used as a chiral selector in CE to separate six basic drugs.15Magnetic microspheres (MNPs) have received significant attention because of their various special characteristics, including high dispersion, high reactivity and easy separation. Over the last few decades, many different compositions and phases of magnetic microspheres have been synthesized by some common methods, such as co-precipitation, thermal decomposition, microemulsion synthesis and hydrothermal synthesis.16–19 Furthermore, magnetic microspheres can be used for further functionalization to be widely applied to a range of disciplines, including environmental remediation, drug delivery, cancer diagnosis and magnetic resonance imaging.20–24 In recent years, several groups have used chiral selector-modified MNPs for separation of chiral compounds. Wei et al. employed MNPs coated with an immobilized cellulose derivative for the research of racemates.25 Our group reported teicoplanin-modified magnetic mesoporous silica microspheres for the absorption of five chiral compounds.26
In this paper, a novel maleic anhydride-β-cyclodextrin (MAH-β-CD) surface-modified magnetic nanosphere is prepared by polymerizing the MAH-β-CD to enantioselective absorbs four enantiomers. Various characterization techniques confirm the presence of MAH-β-CD successful grafted onto the surface of poly(N,N′-methylene bisacrylamide)@Fe3O4 (P(MBAAm)@Fe3O4) microspheres. The specific rotation and CE separation of the residual sample solution after interaction with MAH-β-CD-modified Fe3O4 microspheres indicate the chiral separation ability of MAH-β-CD-modified magnetic microspheres. The enantioselective absorption mechanism is discussed in the following analysis.
2. Materials and methods
2.1 Materials and reagents
Tetraethyl orthosilicate (TEOS) and N,N′-methylene bisacrylamide (MBAAm) were supplied by J&K Scientific (Beijing, China). Maleic anhydride (MAH) and chlorpheniramine maleate salt were purchased from Sigma-Aldrich (St. Louis, MO, USA). The DL-tryptophan, DL-phenylalanine and DL-2-phenylglycine were from Acros Organics (Geel, Belgium). 2,2′-Azobisisobutyronitrile (AIBN) and tris(hydroxymethyl)methyl aminomethane (tris) was obtained from Shanghai Sihe Chemical Co., Ltd. (Shanghai, China). β-Cyclodetrin (β-CD) was purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China), and was purified three times by double distilled water. All other chemical reagents were commercially available at an analytical grade without further purification.2.2 Characterization
The size of the magnetic microspheres was analyzed with a Hitachi H-800 transmission electron microscope (TEM; Tokyo, Japan). Power X-ray diffraction (XRD) measurements were taken using Rigaku Ultima3 (Tokyo, Japan) with Cu Kα radiation (40 kV, 30 mA), under a scan speed of 5° min−1 from 20 to 80°. Fourier transform infrared spectra (FT-IR) were collected using Nicolet 8700 (Thermo Fisher, Waltham, MA, USA). Thermogravimetric analysis (TGA) was carried out on a HCT-2 thermo-analysis system (Beijing Henven Scientific Instrument Corporation, Beijing, China) with a heating rate of 10 °C min−1 from 25 to 800 °C under a N2 flow velocity of 10 ml min−1. The chemical structures of the prepared MAH-β-CD derivatives were confirmed by 13C-NMR spectra using a Bruker Avance-400 spectrometer. The magnetic properties of the microspheres were obtained on a 7410 Lake Shore vibrating sample magnetometer (VSM, Westerville, OH, USA). The elemental results were collected on a vario EL cube (Elementar, Hanau, Germany). Optical activity was measured with a WZZ-2S automatic digital polarimeter (Pudong Optical Instruments, Shanghai, China) at room temperature with a 1 decimeter cell-path.2.3 Synthesis of SiO2@Fe3O4 microspheres
The dispersible SiO2@Fe3O4 microspheres were prepared according to a solvothermal synthesis and the Stöber method with some modification.16,27 In a typical procedure, FeCl3·6H2O (13.5 g) and anhydrous sodium acetate (36 g) were dissolved by ultrasonication in ethylene glycol (400 mL) to form a yellow solution, followed by vigorous magnetic stirring for 1 h. The obtained homogenous yellow solution was sealed in five Teflon-lined autoclaves. The autoclave was placed in 200 °C oven for 8 h, and then cooled to room temperature. The black products were collected and washed with ethanol three times, and then dried under vacuum at 60 °C for 2 h. Next, dry Fe3O4 (1 g) was ultrasonically dispersed in a 500 mL three-necked flask containing 5 mL of ammonia aqueous, 50 mL of water, and 150 mL of ethanol. Then, 1 mL of tetraethyl orthosilicate (TEOS) diluted in ethanol (50 mL) was consecutively added to the mixture solution under vigorous stirring. After 6 h, the obtained magnetic microspheres with silica were washed sequentially with 1 M of HCl and deionized water three times and separated with the help of a magnet. The resulting sample was dried under vacuum at 60 °C for further use.2.4 Preparation of P(MBAAm)@Fe3O4 microspheres
The magnetic nanoparticles with active groups, such as carboxyl groups or a double bond, is necessary to maintain the nanoparticle stability and to increase their application field. The distillation precipitation polymerization approach has been applied to prepare silica/polymer microspheres, because this approach causes the formation of a hydrogen bond between the silica and oligomer.28–30 According to this same the principle, therefore, polymer@Fe3O4 microspheres were prepared. The details are as followed: SiO2@Fe3O4 microspheres (0.2 g) and MBAAm (0.1 g), used respectively as the seeds and the monomer, were ultrasonically dispersed in a 100 mL two-necked flask containing 80 mL of acetonitrile for 10 min. Subsequently, the initiator AIBN (2 mg) was added to the above solution. This mixture solution was slowly heated to 94 °C for 20 min under stirring, and the polymerization was triggered under refluxing. The whole reaction was continued by distilling the solvent out of the flask. The polymerization reaction was ended and cooled when 40 mL of acetonitrile was distilled off. The obtained product was washed with 40 mL ethanol three times under ultrasonication, and then was dried in a vacuum oven at 60 °C for 3 h.2.5 Fabrication of MAH-β-CD-modified Fe3O4 microspheres
The P(MBAAm)@Fe3O4 microspheres (0.2 g) were dispersed in 40 mL of deionized water under ultrasonication for 10 min. Afterwards, 0.04 g of MBAAm and 0.46 g of MAH-β-CD (see the ESI† for the prepared procedures) were dissolved in the above solution in a 50 mL three-necked flask. Under N2 atmosphere, the mixture solution was slowly heated to 90 °C with stirring. Then 5 mL of deionized water containing 20 mg (4 wt% relative to the total monomer) of potassium persulfate was added dropwise to this mixture suspension. After stirring for 3 h at 90 °C, the finished product was collected with the help of a magnet and washed with deionized water three times. Finally, the MAH-β-CD-modified Fe3O4 microspheres were dried in a vacuum oven for 3 h.2.6 Enantioselective absorption of four enantiomers
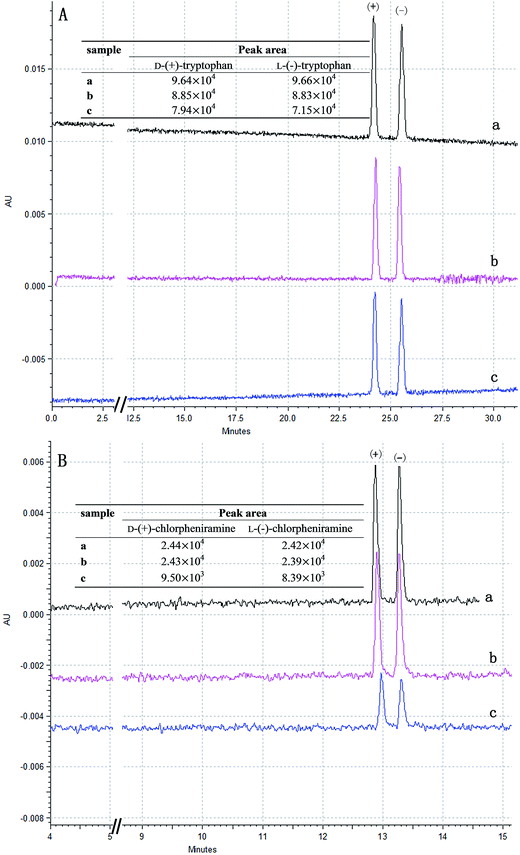
The enantioselective absorption of enantiomers with the MAH-β-CD-modified Fe3O4 microspheres is investigated. The detail processes are as followed: four chiral compounds (2 mg mL−1) were prepared by dissolving the three amino acids and one chiral drug in 10 mL phosphate buffer (20 mM, pH 8) via ultrasonication. Then, the MAH-β-CD-modified Fe3O4 microspheres (100 mg) were dispersed in the same phosphate buffer under ultrasonication. With the help of a magnet, the functional magnetic nano-particles were separated and added to one of the reference samples. The obtained suspension was shaken for 10 min by vortex. Subsequently, the supernatant was collected and determined using an automatic digital polarimeter after magnetic separation. At the same time, the experiments of the phosphate buffer after interaction with MAH-β-CD-modified Fe3O4 microspheres and the elution of the adsorbed analyses from the MAH-β-CD-modified Fe3O4 microspheres after interaction with racemic mixture were also performed to explore the stability of the immobilized selector at the microparticle surface and provide the binding isotherm data. The process of the phosphate buffer after interaction with MAH-β-CD-modified Fe3O4 microspheres was consistent with the enantioselective absorption experiment. The collected microspheres after interaction with racemic mixture were re-dispersed in 5 ml buffer solution and shaken for 10 min by vortex. The elution process was repeated twice and the obtained supernatants were merged to measure using an automatic digital polarimeter. All processes were repeated four times.To further verify the enantioselective absorption results, the residual DL-tryptophan and chlorpheniramine maleate after chiral capture with the magnetic microspheres were analyzed by CE. 0.2 mg mL−1 DL-tryptophan and chlorpheniramine maleate were prepared by diluting the 2 mg mL−1 solution with the same phosphate buffer. Then, 1 mL of diluted sample solution was inserted into a tube containing 100 mg of MAH-β-CD-modified Fe3O4 microspheres. After the interaction between the chiral compound and the chiral modified magnetic microspheres, the residue sample was collected and filtered for CE analysis. The calibration curves of D/L-tryptophan and chlorpheniramine maleate were then constructed by plotting the peak area as a function of the concentration. In order to fit in the linear range of the calibration, the different concentrations were diluted with the buffer. The analysis was performed on a Beckman Coulter MDQ system combined with a UV detector at 214 nm (Beckman Coulter Corp., CA, USA).
3. Results and discussion
The overall experiment process used in this study can is illustrated in Fig. 1. In brief, a facile solvothermal method and sol–gel approaches were sequentially applied to prepare the dispersible SiO2@Fe3O4 microspheres. To be further functionalized with a chiral selector, the P(MBAAm)@Fe3O4 microspheres were prepared by the distillation precipitation polymerization approach, and a double bond was introduced onto the surface of the SiO2@Fe3O4 microspheres. Finally, the MAH-β-CD-modified Fe3O4 microspheres were prepared by free radical polymerization and subsequently used to separate chiral compounds.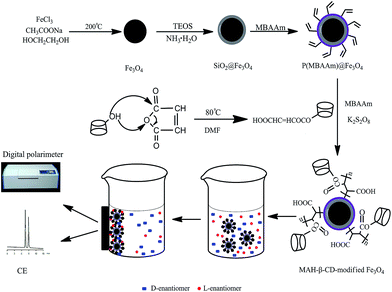 | ||
| Fig. 1 Schematic of the synthetic procedure for the MAH-β-CD-modified Fe3O4 microspheres and the enantioseparation of the compounds. | ||
3.1 Optimization of the potassium persulfate concentration
A suitable initiation concentration can provide a reasonable reaction rate in the polymerization. To further explore the effect of the potassium persulfate concentration during the second-stage polymerization, the potassium persulfate concentration was varied from 2–10 wt% (relative to the total monomer). The elemental results are shown in Table 1. An increase in initiator concentration up to 4 wt% (A1, A2) leads to an increased carbon content, which indicates that an increased amount of MAH-β-CD is being grafted onto the surface of the magnetic microspheres. However, further increase of the initiator (A3, A4) causes carbon content to decrease. One explanation is that a large number of free radicals results in a majority of the monomers being polymerized with themselves and not with the P(MBAAm)@Fe3O4 microspheres. In consideration of this result, the potassium persulfate concentration was chosen to be 4 wt% (relative to the total monomer) for this study.| MAH-β-CD-modified Fe3O4 | Potassium persulfate (wt%) | N% | C% |
|---|---|---|---|
| A1 | 2 | 1.12 | 6.77 |
| A2 | 4 | 1.76 | 9.59 |
| A3 | 6 | 1.52 | 8.76 |
| A4 | 10 | 1.08 | 6.59 |
3.2 Characterization of the synthesized chiral magnetic microspheres
The size and shape of the magnetic microspheres are measured using TEM. From the TEM images, the magnetic microspheres are spherical and have a very narrow diameter distribution. Fig. 2a shows that the Fe3O4 microspheres are almost monodisperse and have a mean diameter of about 450 nm. The core–shell structures can be observed in the TEM images of the SiO2@Fe3O4 microspheres. Fig. 2b distinctly shows that the outer layer thickness of the Fe3O4 microspheres with silica to be about 50 nm. The TEM images of the P(MBAAm)@Fe3O4 microspheres are shown in Fig. 2c, which indicates that the P(MBAAm)@Fe3O4 microspheres have a thick polymer wall with about 10 nm thick. It can be clearly seen that the MAH-β-CD-modified Fe3O4 microspheres have a three-layer structure with a relatively thick chiral polymer layer about 35 nm thick (Fig. 2d). Comparing with Fig. 2c, the thickness of the polymer in Fig. 2d is seen to increase after grafting the MAH-β-CD onto the microspheres, which indicates the successful preparation of MAH-β-CD-modified Fe3O4 microspheres.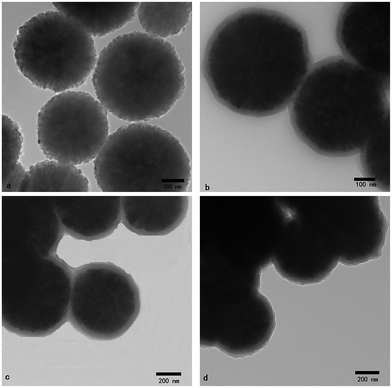 | ||
| Fig. 2 TEM images of (a) Fe3O4, (b) SiO2@Fe3O4, (c) P(MBAAm)@Fe3O4, and (d) MAH-β-CD-modified Fe3O4 microspheres. | ||
The XRD patterns of the as-synthesized samples are shown in Fig. 3. The characterized diffraction peaks of the Fe3O4 microspheres are in good agreement with the standard Fe3O4 (JCPDS, no.85-1436; not shown), and which reveals that the obtained particles are pure Fe3O4 with a typical cubic structure. Using the Debye-Scherrer equation, the mostly crystalline average sizes were calculated to be 16 nm, which indicated that the microspheres of the TEM images are made of a large number of crystalline grains. After sequentially modifying of the Fe3O4 with silica, MBAAm and finally MAH-β-CD, the phase change of the Fe3O4 is not obtained and the intensity of Fe3O4 decreases because the outer layer affects the penetration of X-rays into the core.
 | ||
| Fig. 3 X-ray powder diffraction patterns of (a) Fe3O4, (b) SiO2@Fe3O4, (c) P(MBAAm)@Fe3O4, and (d) MAH-β-CD-modified Fe3O4 microspheres. | ||
To provide further evidence of the presence of immobilized MAH-β-CD on the surface of the magnetic microspheres, the FT-IR spectra of the prepared samples are shown in Fig. 4, with the curves plotted progressing from (a) Fe3O4 microspheres, to (b) SiO2@Fe3O4 microspheres, then to (c) P(MBAAm)@Fe3O4 microspheres, and finally to (d) MAH-β-CD-modified Fe3O4 microspheres. The strong peak at 588 cm−1 evident in curves (a–d) is characteristic of Fe–O vibrations, and the wide absorption peak ranging from 3600 to 3200 cm−1 was attributed to the stretching vibration of O–H bonds. The asymmetric and symmetric stretching of Si–O–Si bonds at 1084 and 796 cm−1 appears in curves (b–d), revealing that the Fe3O4 is coated with silica in these samples. The FT-IR spectra of the P(MBAAm)@Fe3O4 nanosphere sample exhibits the peaks at 2924 and 2847 cm−1 attributed to C–H stretching vibrations, while the new peak at 1530 cm−1 is assigned to the bending vibration of the N–H bond in the P(MBAAm) component. Unlike curve (c) and the spectrum of pure MAH-β-CD, the absorption peak at 1718 cm−1 can be observed in the spectrum of MAH-β-CD-modified Fe3O4 microspheres (curve (d)), which corresponds to the vibration of the carbonyl group in MAH-β-CD. These results further prove that a P(MAH-β-CD) shell is successfully coated onto the magnetic microspheres through the second-stage polymerization.
 | ||
| Fig. 4 FT-IR spectra of (a) Fe3O4, (b) SiO2@Fe3O4, (c) P(MBAAm)@Fe3O4, (d) MAH-β-CD-modified Fe3O4 microspheres and (e) MAH-β-CD. | ||
The magnetization saturation of the microspheres is important for fast separation, experimental repeatability and application. Therefore, the magnetic properties of the samples are measured at room temperature by VSM, and the magnetization curves of the four different types of coated and uncoated Fe3O4 microspheres can be clearly observed in Fig. 5, revealing no obvious magnetic hysteresis loops. The saturation magnetization values for Fe3O4, SiO2@Fe3O4, P(MBAAm)@Fe3O4, and MAH-β-CD-modified Fe3O4 microspheres are 82.4, 66.5, 65.1 and 60.3 emu g−1, respectively. Because of the weight contribution from the nonmagnetic inorganic and organic components, the thickness of the shell layer results in a decrease in the magnetic strength, especially for the SiO2@Fe3O4 and MAH-β-CD-modified Fe3O4 microspheres. However, the magnetism of these microspheres is still strong enough to be measured. As shown in the inset of Fig. 5, the MAH-β-CD-modified Fe3O4 microspheres dispersed in deionized water results in a black suspension by sonication, and are easily collected from the mixture solution by a relatively low external magnetic field. These results indicate that the chiral magnetic microspheres have some magnetic responsiveness, which is good for use in practical research.
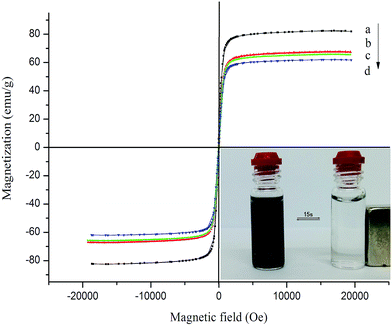 | ||
| Fig. 5 Magnetization curves of (a) Fe3O4, (b) SiO2@Fe3O4, (c) P(MBAAm)@Fe3O4, and (d) MAH-β-CD-modified Fe3O4 microspheres. | ||
Thermogravimetric analysis is used to investigate the organic content on the surface of the prepared samples, and especially to measure the amount of MAH-β-CD grafted onto the surface of magnetic microspheres, which will affect the enantioselective absorption of chiral compounds. The TGA results are shown in Fig. 6. The TGA curve of the SiO2@Fe3O4 microspheres presents a total weight loss over the full temperature range, which can be explained by the evaporation of the absorbed water or ethanol as well as dehydration of the OH group from the nanosphere surface. The TGA curve for P(MBAAm)@Fe3O4 microspheres exhibits a small drop from the level of the SiO2@Fe3O4 nanosphere curve due to the thermal decomposition of the P(MBAAm), which indicates that the surface of the P(MBAAm)@Fe3O4 microspheres has a thin layer of organic material. The TGA curve of MAH-β-CD-modified Fe3O4 microspheres exhibits a drastic drop compared with the previous two curves, which is mainly due to the thermal decomposition of the P(MAH-β-CD). This also confirms that a large amount of MAH-β-CD is grafted onto the surface of microspheres. In this way, TGA curves can convey information about the amount of P(MAH-β-CD) on the microspheres. Elemental analysis results from Table 2 indicate that The amount of MAH-β-CD modified on the P(MBAAm)@Fe3O4 was about 149.1 mg g−1. This result is consistent with the TGA curves.
 | ||
| Fig. 6 TGA curves of (a) SiO2@Fe3O4, (b) P(MBAAm)@Fe3O4, and (c) MAH-β-CD-modified Fe3O4 microspheres. | ||
| Sample | N% | C% |
|---|---|---|
| SiO2@Fe3O4 | 0.08 | 0.51 |
| P(MBAAm)@Fe3O4 | 0.68 | 2.86 |
| MAH-β-CD-modified Fe3O4 | 1.76 | 9.59 |
3.3 Enantioselective absorption of enantiomers via MAH-β-CD-modified Fe3O4 microspheres
The enantioselective absorption of four enantiomers is used to study the enantioselective capacity of the MAH-β-CD-modified Fe3O4 microspheres. The experimental absorption process is schematically illustrated in Fig. 1, and the correlative results are shown in Table 3. As shown in the results, the specific rotation for a pure racemic mixture is zero because of the equivalent opposite optical rotation, and the optical rotation of the supernatant for the phosphate buffer after interaction with MAH-β-CD-modified Fe3O4 microspheres has only a tiny increase, which showed that there was no optical active substance in the supernatant from the microspheres surface. However, after enantioselective absorption with the MAH-β-CD-modified Fe3O4 microspheres, the specific rotation of the residual solution for four enantiomers is obversed to change. The results of optical rotation attributed to the change in the relative proportion of the (−)-enantiomers and (+)-enantiomers in solution after interaction with the MAH-β-CD-modified Fe3O4 microspheres. In addition, all of the results are positive, which indicates that the MAH-β-CD-modified Fe3O4 microspheres show stronger complexation of (−)-enantiomers than (+)-enantiomers. The reason for this may be that the MAH-β-CD on the Fe3O4 microspheres interacts with the four enantiomers via dipole-dipole interactions, hydrogen bonds and the cavity resulting in the inclusion complex; and the stability constants of these inclusion complexes may be different. In addition, the specific rotation data of tryptophan and chlorpheniramine reveals that their stability constants may be larger because of the double-ring structure, which can be matched with the cavity of MAH-β-CD and create more dipole-dipole interactions.31–34 The elution experiment displayed that the specific rotation was negative, which also indicated that the MAH-β-CD-modified Fe3O4 microspheres have a stronger interaction with (−)-enantiomers than (+)-enantiomers.35| The specific rotation([α]25D) | ||||
|---|---|---|---|---|
| a | b | c | d | |
| a 2 mg mL−1 racemic mixture in 10 mL phosphate buffer before interaction with MAH-β-CD-modified Fe3O4 microspheres.b 10 mL phosphate buffer (20 mM, pH 8) after interaction with MAH-β-CD-modified Fe3O4 microspheres.c The residual solution after enantioselective absorption with chiral microspheres.d Eluted supernatant with phosphate buffer (20 mM, pH 8) from the MAH-β-CD-modified Fe3O4 microspheres after interaction with racemic mixture. | ||||
| DL-Tryptophan | 0.000 | 0.004 | 13.4 | −8.7 |
| DL-Phenylalanine | 0.000 | 0.006 | 6.7 | −5.2 |
| DL-2-Phenylglycine | 0.000 | 0.005 | 7.1 | −5.2 |
| Chlorpheniramine | 0.000 | 0.004 | 15.7 | −10.1 |
To further explore the enantioselective absorption of chiral compounds, the results are further analyzed by capillary electrophoresis (CE). Compared with the specific rotation, the peak area of the enantiomers can directly evaluate the enantioselective absorption of the MAH-β-CD-modified Fe3O4 microspheres. As shown in Fig. 7, the peak areas of the enantiomers are initially equal. After interaction with the P(MBAAm)@Fe3O4 microspheres, tryptophan shows an obvious reduction in the peak area, but the chlorpheniramine does not change much. The reason may be that the P(MBAAm)@Fe3O4 microspheres can form the hydrogen bond through the amino and carboxyl group of the tryptophan. The peak areas of both chiral compounds have a significant reduction but the decrease is different after the interaction with MAH-β-CD-modified Fe3O4 microspheres, which indicates that the MAH-β-CD-modified Fe3O4 microspheres had the chiral discrimination for the two compounds. These results reveal that the MAH-β-CD-modified Fe3O4 microspheres have a stronger interaction with the (−)-enantiomer than the (+)-enantiomer from the peak area of Fig. 7, which is in agreement with the result obtained by specific rotation. Compared with the tryptophan, the peak area of the chlorpheniramine has a sharp reduction, which may be from the chlorpheniramine penetrated into MAH-β-CD cavity to form good inclusion complexation. The calibration curves of D-tryptophan and L-tryptophan were calculated as follows: y = 4.14 × 105x + 1.37 × 104, r = 0.9996 for D-tryptophan; y = 4.21 × 105x + 1.43 × 104, r = 0.9990 for L-tryptophan. The calibration curves of chlorpheniramine were calculated as follows: y = 1.08 × 105x + 2.63 × 103, r = 0.9983 for (+)-enantiomer; y = 1.03 × 105x + 3.76 × 103, r = 0.9993 for (−)-enantiomer. The enantiomeric excesses (e.e.) of DL-tryptophan and chlorpheniramine after adsorption onto MAH-β-CD-modified Fe3O4 microspheres were 12.6% and 13.7%, respectively. Compared with the previous others work, the obtained enantiomeric excesses in our research were accepted.36,37 The specific rotation and CE separation suggest that the MAH-β-CD-modified Fe3O4 microspheres are an effective chiral selector and have some chiral chiral discrimination for the enantiomers.
4. Conclusions
In this study, a facile and convenient polymerization method for the preparation of MAH-β-CD-modified Fe3O4 microspheres as a chiral selector is applied to enantioselective absorb four chiral compounds. The MAH-β-CD-modified Fe3O4 microspheres are characterized by various techniques, which show a three-layer structure and high saturation magnetization. The enantioselective absorption of four chiral compounds further indicates that the MAH-β-CD-modified Fe3O4 microspheres are successfully prepared and have some chiral absorption ability. In addition, the MAH-β-CD-modified Fe3O4 microspheres are found to have stronger enantioselective absorption for double-ring chiral compounds. These chiral magnetic selectors are candidates for future research on the on-line complete separation of chiral compounds by various special techniques and methods in capillary electrophoresis.Acknowledgements
This study was supported by the National Natural Science Foundation of China (no. 21075008), and Beijing Natural Science Foundation (no. 2132048).References
- A. Marley and D. Connolly, J. Chromatogr. A, 2014, 1325, 213–220 CrossRef CAS PubMed.
- A. Rocco, Z. Aturki and S. Fanali, TrAC, Trends Anal. Chem., 2013, 52, 206–225 CrossRef CAS PubMed.
- V. Pérez-Fernández, M.Á García and M. L. Marina, J. Chromatogr. A, 2011, 1218, 6561–6582 CrossRef PubMed.
- J. Ye, M. R. Zhao, J. Liu and W. P. Liu, Environ. Pollut., 2010, 158, 2371–2383 CrossRef CAS PubMed.
- A. Cavazzini, L. Pasti, A. Massi, N. Marchetti and F. Dondi, Anal. Chim. Acta, 2011, 706, 205–222 CrossRef CAS PubMed.
- L. B. Rasmussen, K. H. Olsen and S. S. Johansen, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2006, 842, 136–141 CrossRef CAS PubMed.
- B. Preinerstorfer, M. Lämmerhofer and W. Lindner, Electrophoresis, 2009, 30, 100–132 CrossRef CAS PubMed.
- C. L. Wang and Y. R. Zhang, J. Chromatogr. A, 2013, 1281, 127–134 CrossRef CAS PubMed.
- T. J. Ward and K. D. Ward, Anal. Chem., 2010, 82, 4712–4722 CrossRef CAS PubMed.
- H. Hirai and Y. Shiraishi, React. Funct. Polym., 2007, 67, 1115–1128 CrossRef CAS PubMed.
- B. Chankvetadze, Electrophoresis, 2009, 30, S211–S221 CrossRef PubMed.
- L. Szente and J. Szemán, Anal. Chem., 2013, 85, 8024–8030 CrossRef CAS PubMed.
- D. A. Tsioupi, R.-I. Stefan-Vanstaden and C. P. Kapnissi-Christodoulou, Electrophoresis, 2013, 34, 178–204 CrossRef CAS PubMed.
- Y. Q. Lv, T. C. Hughes, X. J. Hao, D. P. Mei and T. W. Tan, J. Sep. Sci., 2011, 34, 2131–2137 CrossRef CAS PubMed.
- Y. L. Yang, C. F. Zhu, J. Shen and A. Y. Hao, Anal. Sci., 2009, 25, 1315–1318 CrossRef CAS.
- S. H. Im, T. Herricks, Y. T. Lee and Y. N. Xia, Chem. Phys. Lett., 2005, 401, 19–23 CrossRef CAS PubMed.
- H. Iida, K. Takayanagi, T. Nakanishi and T. Osaka, J. Colloid Interface Sci., 2007, 314, 274–280 CrossRef CAS PubMed.
- H. Deng, X. L. Li, Q. Peng, X. Wang, J. P. Chen and Y. D. Li, Angew. Chem., Int. Ed., 2005, 44, 2782–2785 CrossRef CAS PubMed.
- J. Chen, F. B. Wang, K. L. Huang, Y. N. Liu and S. P. Liu, J. Alloys Compd., 2009, 475, 898–902 CrossRef CAS PubMed.
- B. F. Zou, Y. F. Liu and Y. Q. Wang, RSC Adv., 2013, 3, 23327–23334 RSC.
- M. Monier, D. M. Ayad, Y. Wei and A. A. Sarhan, React. Funct. Polym., 2010, 70, 257–266 CrossRef CAS PubMed.
- J. Y. Chung, E. T. Hwang, H. M. Gang and M. B. Gu, React. Funct. Polym., 2013, 73, 39–45 CrossRef CAS PubMed.
- A. Chan, R. P. Orme, R. A. Fricker and P. Roach, Adv. Drug Delivery Rev., 2013, 65, 497–514 CrossRef CAS PubMed.
- M. Bañobre-López, A. Teijeiro and J. Rivas, Rep. Pract. Oncol. Radiother., 2013, 18, 397–400 CrossRef PubMed.
- Y. Wei, A. L. Tian, Y. Li, X. Wang and B. Cao, J. Mater. Chem., 2012, 22, 8499–8504 RSC.
- J. W. Wu, P. Su, J. Huang, S. M. Wang and Y. Yang, J. Colloid Interface Sci., 2013, 399, 107–114 CrossRef CAS PubMed.
- D. Yang, J. H. Hu and S. K. Fu, J. Phys. Chem. C, 2009, 113, 7646–7651 CAS.
- G. Y. Liu, X. L. Yang and Y. M. Wang, Langmuir, 2008, 24, 5485–5491 CrossRef CAS PubMed.
- G. Y. Liu, X. L. Yang and Y. M. Wang, Polymer, 2007, 48, 4385–4392 CrossRef CAS PubMed.
- G. Y. Liu, L. Y. Li and X. L. Yang, Polymer, 2008, 49, 4776–4783 CrossRef CAS PubMed.
- S. Samakashvili, A. Salgado, G. K. E. Scriba and B. Chankvetadze, Chirality, 2013, 25, 79–88 CrossRef CAS PubMed.
- K. Lomsadze, E. D. Vega, A. Salgado, A. L. Crego, G. K. E. Scriba, M. L. Marina and B. Chankvetadze, Electrophoresis, 2012, 33, 1637–1647 CrossRef CAS PubMed.
- V. Šolínová, V. Kašička, P. Sázelová and A. Holý, Electrophoresis, 2009, 30, 2245–2254 CrossRef PubMed.
- M. Blanco and I. Valverde, TrAC, Trends Anal. Chem., 2003, 22, 428–439 CrossRef CAS.
- A. P. Kumar, J. H. Kim, T. D. Thanh and Y. I. Lee, J. Mater. Chem. B, 2013, 1, 4909–4915 RSC.
- J. W. Wu, P. Su, D. H. Guo, J. Huang and Y. Yang, New J. Chem., 2014, 38, 3630–3636 RSC.
- Y. Fu, T. T. Huang, B. Chen, J. Shen, X. L. Duan, J. L. Zhang and W. Li, Sep. Purif. Technol., 2013, 107, 11–18 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 13C-NMR spectrum of MAH-β-CD monomer. See DOI: 10.1039/c4ra12133c |
| This journal is © The Royal Society of Chemistry 2014 |