Synthesis of indoles via dehydrogenative N-heterocyclization by supported platinum catalysts†
Sondomoyee Konika Moromia,
Abeda Sultana Touchya,
S. M. A. Hakim Siddikib,
Md. Ayub Alia and
Ken-ichi Shimizu*ab
aCatalysis Research Center, Hokkaido University, N-21, W-10, Sapporo 001-0021, Japan. E-mail: kshimizu@cat.hokudai.ac.jp; Fax: +81-11-706-9163
bElements Strategy Initiative for Catalysts and Batteries, Kyoto University, Katsura, Kyoto 615-8520, Japan
First published on 25th November 2014
Abstract
We found the first heterogeneous Pt catalysts (Pt/Nb2O5 and Pt/HBEA) for the synthesis of indoles via acceptorless dehydrogenative cyclization of 2-(2-aminophenyl)ethanol, showing higher turnover number (TON) than previously reported catalysts.
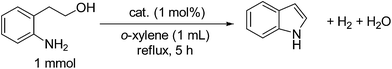
Indoles are important organic compounds, which are widely used for the syntheses of pharmaceuticals and agrochemicals.1–4 Various methods to prepare indoles were reported.1–6 Oxidative or dehydrogenative N-heterocyclization of 2-(2-aminophenyl)ethanol alcohols is one of the most promising protocols,7–17 since the starting alcohol is easily prepared by the condensation of 2-nitrotoluene with formaldehyde in the presence of bases, followed by the reduction of a nitro functionality to an amino one.8 Heterogeneous copper catalysts were reported to catalyze the reaction at high temperatures (>200 °C).7 Homogeneous transition metal catalysts, such as ruthenium8–10 and iridium11–14 complexes and a copper/TEMPO system15 catalyzed the reaction at lower temperatures but have problems such as difficulties in catalyst/product separation and catalyst reuse and needs of additives such as base or acceptor. A supported Au catalyst also required basic additives (200 mol% of NaOtBu) and oxidant (O2).16 From a viewpoint of sustainable chemistry, acceptorless dehydrogenative coupling methodology5,6,18–20 under neutral conditions using a reusable catalyst is most attractive. Recently, Wada and co-workers17 developed a new heterogeneous catalyst, Ru/CeO2, that was effective for acceptorless dehydrogenative synthesis of indole from 2-(2-aminophenyl)ethanol in the absence of any additives. As discussed in the previous studies on this reaction, the reaction can proceeds via the following two step pathway:
where dehydrogenation of 2-(2-aminophenyl)ethanol by a metal (M) catalyst is followed by condensation of the amino aldehyde intermediate to give indole. As a part of our continuing interest in heterogeneous Pt catalysts for acceptorless dehydrogenation of alcohols21 and acceptorless dehydrogenative coupling reactions of alcohols,22,23 we report herein the synthesis of indoles via acceptorless dehydrogenative N-heterocyclization of 2-(2-aminophenyl)ethanol under additive-free conditions by heterogeneous Pt catalysts, which show higher TON than previous catalytic systems.
We started the N-heterocyclization of 2-(2-aminophenyl)ethanol under o-xylene reflux conditions in N2 as a model system in order to optimize catalytic conditions. Table 1 (entries 1–8) summarizes the results of the initial catalyst screening test under the same reaction conditions using various transition metal (Pt, Ir, Ru, Re, Pd, Co, Cu, Ag) catalysts supported on Nb2O5 pre-reduced in H2 at 300 °C. Cu and Ag catalysts (entries 7 and 8) showed no activity and Ru, Re, Pd and Co catalysts (entries 3–6) showed low yields. Ir/Nb2O5 (entry 2) gave a good yield of 68%. Among the catalysts tested, Pt/Nb2O5 (entry 1) showed the highest yield (82%) of indole. Entries 10–19 show the results of Pt catalysts loaded on the other supports (HBEA zeolite, CeO2, MgO, TiO2, ZrO2, Al2O3, SnO2, carbon, SiO2). Pt/Nb2O5 (entry 1) and Pt/HBEA (entry 10) showed higher yields (82–83%) than the other supported Pt catalysts. Pt/CeO2, Pt/MgO, Pt/TiO2, Pt/ZrO2, Pt/Al2O3, Pt/SiO2–Al2O3, Pt/C and Pt/SiO2 (entries 11–19) gave low to moderate yields (9–69%). To study the effect of the oxidation state of Pt species on the activity, we tested the activity of a pre-oxidized catalyst named PtOx/Nb2O5, that is, a platinum oxides-loaded Nb2O5 catalyst. PtOx/Nb2O5 (entry 20) showed a lower yield (10%) than the pre-reduced catalyst, Pt/Nb2O5 (entry 1). Taking into account the result that the activity of the metal-unloaded Nb2O5 is negligible (entry 9) and the result that the activity depends strongly on the support, it is suggested that the co-presence of metallic Pt species and specific support materials (Nb2O5 or HBEA) is important factor of highly active catalysts. Note that indole was not produced in the absence of a catalyst (entry 21).
| Entry | Catalyst | Yielda (%) |
|---|---|---|
| a Yield was determined by GC.b Catalyst amount was 39 mg. | ||
| 1 | Pt/Nb2O5 | 82 |
| 2 | Ir/Nb2O5 | 68 |
| 3 | Ru/Nb2O5 | 9 |
| 4 | Re/Nb2O5 | 2 |
| 5 | Pd/Nb2O5 | 1 |
| 6 | Co/Nb2O5 | 1 |
| 7 | Cu/Nb2O5 | 0 |
| 8 | Ag/Nb2O5 | 0 |
| 9b | Nb2O5 | 1 |
| 10 | Pt/HBEA | 77 |
| 11 | Pt/CeO2 | 69 |
| 12 | Pt/MgO | 69 |
| 13 | Pt/TiO2 | 47 |
| 14 | Pt/ZrO2 | 42 |
| 15 | Pt/Al2O3 | 22 |
| 16 | Pt/SiO2–Al2O3 | 15 |
| 17 | Pt/SnO2 | 9 |
| 18 | Pt/C | 15 |
| 19 | Pt/SiO2 | 10 |
| 20 | PtOx/Nb2O5 | 10 |
| 21 | Blank | 0 |
Table 2 shows the effect of reaction conditions on the yield of indole for the reaction of 2-(2-aminophenyl)ethanol by Pt/Nb2O5 under reflux conditions for 7 h. The reactions in toluene and hexane gave low yield (entries 3 and 4), and the reaction in mesitylene gave 78% yield (entry 2). The reaction in reflux of o-xylene gave the highest yield of 93% (entry 1). After the reaction, the catalyst was removed from the mixture and indole was isolated by column chromatography, resulting in high isolated yield of indoles (88%). The reaction in o-xylene at lower temperature (130 °C) gave low yield (29%) of indole (entry 5). The reaction under 1 atm O2 in the gas phase (entry 6) gave lower yield (82%) than that under N2 (93%), indicating that O2 as a hydrogen acceptor did not accelerate the catalytic reaction. For the reaction under N2 with Pt/Nb2O5, we carried out mass spectrometry analysis of the gas phase products after 1 h. As shown in eqn (1), the yields of gas phase H2 (24%) was close to the yield of indole (32%).
Using Pt/Nb2O5 and Pt/HBEA as two of the effective catalysts for this reaction, we carried out detailed catalytic studies. We checked the time course of the reaction under the standard conditions (Fig. 1). For Pt/Nb2O5, the yield of indole increased with time and reached 93% after 7 h. For Pt/HBEA, the yield of indole reached 95% after 12 h. Fig. 2 shows the results of catalyst reusability of Pt/Nb2O5 and Pt/HBEA. After completion of the reaction, 2-propanol (1 mL) was added to the reaction mixture and the catalyst was separated by centrifugation. The recovered catalyst was washed with acetone three times, followed by centrifugation and drying in an oven (under air) at 90 °C for 12 h and then H2 reduction at 300 °C for 0.5 h. The recovered Pt/Nb2O5 catalyst showed high yield for the second and the third cycle. In contrast, the recovered Pt/HBEA catalyst showed low yields in the second and third cycles. Thus, Pt/Nb2O5 was found to be a better catalyst in terms of reusability.
 | ||
| Fig. 1 Yields of indole vs. time for N-heterocyclization of 2-(2-aminophenyl)ethanol under o-xylene reflux conditions in N2 by Pt/Nb2O5 (○) or Pt/HBEA (△). | ||
 | ||
| Fig. 2 Reuse of Pt/Nb2O5 (gray bars) or Pt/HBEA (black bars) for N-heterocyclization of 2-(2-aminophenyl)ethanol under o-xylene reflux conditions in N2 for 7 h. | ||
In order to evaluate TON of the catalytic system, next we carried out the reaction with small amount of the catalysts. As shown in eqn (2), the reactions with 0.2 mol% of Pt/Nb2O5 and Pt/HBEA for 52 h resulted in 76% and 90% yields, corresponding to TONs of 380 and 450, respectively. The TON by Pt/HBEA is 3–25 times larger than those by homogeneous8–15 and heterogeneous17 catalysts in the literature for the same reaction. ICP-AES analysis of the filtrate after the reaction with Pt/HBEA showed that the content of Pt in the solution was quite low (3.4 ppm).
The present method was also effective for the synthesis of a functionalized indole. As shown in eqn (3), the 2-aminophenethyl alcohol with a Cl-group on the aromatic ring was converted to the corresponding indole derivative in 71% isolated yield by 1 mol% of Pt/HBEA.
Since supported Pt catalysts can exhibit a catalytic activity for the reduction of the nitro group to an amino group with H2, we examined the synthesis of indoles from 2-nitrophenthyl alcohols. As shown in eqn (4) and (5), Cl-functionalized 2-nitrophenthyl alcohol and 2-nitrophenthyl alcohol were selectively converted to the corresponding indole derivatives in moderate isolated yields under balloon H2 pressure for 12 h in the presence of 1 mol% of Pt/HBEA.
In summary, we reported that Pt/Nb2O5 and Pt/HBEA acted as effective heterogeneous catalysts for the synthesis of indoles via acceptorless dehydrogenative cyclization of 2-(2-aminophenyl)ethanol. These catalysts showed higher TON than previously reported catalysts, and the Pt/Nb2O5 catalyst was reusable.
Experimental section
Commercially available organic compounds (from Tokyo Chemical Industry) were used without further purification. The GC (Shimadzu GC-14B) and GCMS (Shimadzu GCMS-QP2010) analyses were carried out with Ultra ALLOY capillary column UA+-1 (Frontier Laboratories Ltd.) using nitrogen and helium as the carrier gas.H+-type BEA zeolite (HBEA, SiO2/Al2O3 = 25 ± 5, JRC-Z-HB25), CeO2 (JRC-CEO-3), MgO (JRC-MGO-3), TiO2 (JRC-TIO-4) and SiO2–Al2O3 (JRC-SAL-2, Al2O3 = 13.75 wt%) were supplied from Catalysis Society of Japan. Nb2O5 was prepared by calcination of niobic acid (CBMMI) at 500 °C for 3 h. SnO2 was prepared by calcination of H2SnO3 (Kojundo Chemical Laboratory Co., Ltd.) at 500 °C for 3 h. ZrO2 was prepared by calcination of a hydroxide of Zr at 500 °C for 3 h.23 γ-Al2O3 was prepared by calcination of γ-AlOOH (Catapal B Alumina purchased from Sasol) at 900 °C for 3 h. Precursor of 5 wt% Pt/Nb2O5 was prepared by an impregnation method; a mixture of Nb2O5 and an aqueous HNO3 solution of Pt(NH3)2(NO3)2 was evaporated at 50 °C, followed by drying at 90 °C for 12 h. A pre-reduced catalyst, named Pt/Nb2O5, was prepared by pre-reduction of the precursor in a Pyrex tube under a flow of H2 (20 cm3 min−1) at 300 °C for 0.5 h. Platinum oxides-loaded Nb2O5 (PtOx/Nb2O5), as a comparative catalyst, was prepared by calcination of the precursor in air at 300 °C for 3 h. By using various supports, several pre-reduced Pt catalysts were prepared by the same method as Pt/Nb2O5. Nb2O5-supported metal catalysts, M/Nb2O5 (M = Co, Cu, Ru, Pd, Ag, Re, Ir) with metal loading of 5 wt% were prepared by impregnation method in a similar manner as Pt/Nb2O5 using an aqueous solution of metal nitrates (for Co, Cu, Ag), RuCl3, IrCl3, NH4ReO4 or an aqueous HNO3 solution of Pd(NO3)2.
Typically, 5 wt% Pt/Nb2O5 (39 mg, 0.01 mmol of Pt) was used as a standard catalyst. After the pre-reduction at 300 °C, we carried out catalytic tests using a batch-type reactor without exposing the catalyst to air as follows. A mixture of 2-(2-aminophenyl)ethanol (1.0 mmol) and n-dodecane (0.2 mmol) in o-xylene (1 mL) was injected to the pre-reduced catalyst inside the reactor (cylindrical glass tube) through a septum inlet, followed by filling N2. Then, the resulting mixture was magnetically stirred for 5 h under reflux condition; the bath temperature was 155 °C and reaction temperature was ca. 144 °C. The yield of indole was determined by GC using n-dodecane as an internal standard. The analysis of the gas phase product (H2) was carried out by the mass spectrometer (BELMASS). To determine the isolated yield of indole, indole was isolated by column chromatography using silica gel 60 (spherical, 63–210 μm, Kanto Chemical Co. Ltd.) with hexane/ethylacetate (95/5) as the eluting solvent, followed by analyses by GCMS and 1H and 13C NMR (JEOL-ECX 600 operating at 600.17 and 150.92 MHz, respectively) with tetramethylsilane as an internal standard.
Acknowledgements
This work was supported by Grant-in-Aids for Scientific Research B (26289299) from MEXT (Japan), a MEXT program “Elements Strategy Initiative to Form Core Research Center” and a Grant-in-Aid for Scientific Research on Innovative Areas “Nano Informatics” (25106010) from JSPS.Notes and references
- (a) R. J. Sundberg, The Chemistry of Indoles, Academic Press, New York, 1970 Search PubMed; (b) L. S. Hegedus, Angew. Chem., Int. Ed., 1988, 27, 1113–1226 CrossRef; (c) T. L. Gilchrist, J. Chem. Soc., Perkin Trans. 1, 1999, 2849–2866 RSC.
- G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045–1075 RSC.
- S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873–2920 CrossRef CAS PubMed.
- J. Barluenga, F. Rodríguez and F. T. Fañanás, Chem.–Asian J., 2009, 4, 1036–1048 CrossRef CAS PubMed.
- M. Zhang, F. Xie, X. T. Wang, F. Yan, T. Wang, M. Chen and Y. Ding, RSC Adv., 2013, 3, 6022–6029 RSC.
- M. Tursky, L. L. R. Lorentz-Petersen, L. B. Olsen and R. Madsen, Org. Biomol. Chem., 2010, 8, 5576–5582 CAS.
- W. Hammerschmidt, A. Baiker, A. Wokaun and W. Fluhr, Appl. Catal., 1986, 20, 305–312 CrossRef CAS.
- Y. Tsuji, S. Kotachi, K.-T. Huh and Y. Watanabe, J. Org. Chem., 1990, 55, 580–584 CrossRef CAS.
- T. Kondo, T. Kanda, D. Takagi, K. Wada, Y. Kimura and A. Toshimitsu, J. Heterocycl. Chem., 2012, 86, 1015–1022 CAS.
- S. C. Ghosh and S. H. Hong, Eur. J. Org. Chem., 2010, 4266–4270 CrossRef CAS.
- K. Fujita, K. Yamamoto and R. Yamaguchi, Org. Lett., 2002, 4, 2691–2694 CrossRef CAS PubMed.
- S. Whitney, R. Grigg, A. Derrick and A. Keep, Org. Lett., 2007, 9, 3299–3302 CrossRef CAS PubMed.
- C.-F. Fu, Y.-H. Chang, Y.-H. Liu, S.-M. Peng, C. J. Elsevier, J.-T. Chen and S.-T. Liu, Dalton Trans., 2009, 6991–6998 RSC.
- G. Guisado-Barrios, J. Hiller and E. Peris, Chem.–Eur. J., 2013, 19, 10405–10411 CrossRef CAS PubMed.
- J. C. A. Flanagan, L. M. Dornan, M. G. McLaughlin, N. G. McCreanor, M. J. Cook and M. J. Muldoon, Green Chem., 2012, 14, 1281–1283 RSC.
- R. Bernini, S. Cacchi, G. Fabrizi, S. Niembro, A. Prastaro, A. Shafir and A. Vallribera, ChemSusChem, 2009, 2, 1036–1040 CrossRef CAS PubMed.
- S. Shimura, H. Miura, K. Wada, S. Hosokawa, S. Yamazoe and M. Inoue, Catal. Sci. Technol., 2011, 1, 1340–1346 CAS.
- (a) C. Gunanathan and D. Milstein, Science, 2013, 341, 249–260 CrossRef CAS PubMed; (b) G. E. Dobereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681–703 CrossRef CAS PubMed; (c) C. Chen and S. Hyeok Hong, Org. Biomol. Chem., 2011, 9, 20–26 RSC; (d) T. Hille, T. Irrgang and R. Kempe, Chem.–Eur. J., 2014, 20, 5569–5572 CrossRef CAS PubMed; (e) S. Michlik and R. Kempe, Nat. Chem., 2013, 5, 140–144 CrossRef CAS PubMed; (f) F. Xie, M. Chen, X. Wang, H. Jiang and M. Zhang, Org. Biomol. Chem., 2014, 12, 2761–2768 RSC.
- H. Wang, J. Zhang, Y.-M. Cui, K.-F. Yang, Z.-J. Zheng and L.-W. Xu, RSC Adv., 2014, 4, 34681–34686 RSC.
- X. Wang, M. Chen, F. Xie and M. Zhang, RSC Adv., 2014, 4, 14744–14751 RSC.
- K. Kon, S. M. A. H. Siddiki and K. Shimizu, J. Catal., 2013, 304, 63–71 CrossRef CAS PubMed.
- S. M. A. H. Siddiki, K. Kon, A. S. Touchy and K. Shimizu, Catal. Sci. Technol., 2014, 4, 1716–1719 Search PubMed.
- S. K. Moromi, S. M. A. H. Siddiki, M. A. Ali, K. Kon and K. Shimizu, Catal. Sci. Technol., 2014, 4, 3631–3635 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra11893f |
| This journal is © The Royal Society of Chemistry 2015 |