Low temperature growth of hybrid ZnO/TiO2 nano-sculptured foxtail-structures for dye-sensitized solar cells†
Abstract
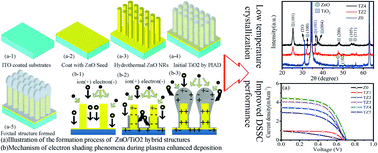
ZnO/TiO2 nano-sculptured foxtail-structures were fabricated using two sequential low-temperature processes combining hydrothermal growth of ZnO nanorods (NRs) and plasma ion assisted evaporation of crystalline TiO2 nanostructures. The ZnO NRs were homogeneously covered with a thin layer of anatase TiO2 nanostructure to form nano-sculptured foxtail-like patterns. Power conversion efficiency of dye-sensitized solar cells made from these hybrid ZnO/TiO2 structures was improved from 0.3% to 1.8% after using the ZnO/TiO2 hybrid structure. Measurement using electrochemical impedance spectroscopy and photocurrent decay proved that the hybrid structures have good electron transport capability, because the ZnO NRs can provide a direct pathway for charge transport; the TiO2 layer can improve charge injection and prevent the formation of Zn2+/dye complex (thus reducing the recombination); the hybrid structures can further increase surface area (thus higher dye loading).

 Please wait while we load your content...
Please wait while we load your content...