Atom efficient thermal and photocuring combined treatments for the synthesis of novel eco-friendly grid-like zein nanofibres
Qingqing Wanga,
Avinav G. Nandgaonkarbc,
Jing Cuia,
Fenglin Huanga,
Wendy E. Krausec,
Lucian A. Lucia*bcd and
Qufu Wei*a
aKey Laboratory of Eco-Textiles, Ministry of Education, Jiangnan University, 1800 Lihi Avenue, Jiangsu Province, Wuxi 214122, P.R. China. E-mail: qfwei@jiangnan.edu.cn
bState Key Lab of Pulp & Paper Science and Technology of the Ministry of Education, Qilu University of Technology, Jinan, 250353, Shandong, P.R. China. E-mail: lucian.lucia@gmail.com
cFiber and Polymer Science Program, North Carolina State University, Raleigh, NC 27695, USA
dThe Laboratory of Soft Materials & Green Chemistry, Departments of Chemistry, Wood & Paper Science (Forest Biomaterials), North Carolina State University, Raleigh, NC 27695, USA
First published on 3rd November 2014
Abstract
We report herein for the first time a novel crosslinking approach for the synthesis of grid-like zein nanofibres with SbQ (styrylpyridinequaternary) realized by a simple electrospinning process followed by thermal treatment and/or UV illumination. The properties of the electrospinning solution, such as viscosity, conductivity, and surface tension, were tested to evaluate the effect of SbQ addition (0 wt%, 10 wt%, 20 wt%) on the electrospinnability of glacial acetic acid solution of zein (25 wt%). The incorporation of SbQ resulted in bead-free nanofibre structures with increased diameter compared to pure zein nanofibres. The FT-IR results indicated that the zein-glacial acidic acid protein solution crosslinked, a phenomenon that can be characterized by two discrete, temporally distinct events: inter-molecular solution crosslinking and intra-fiber crosslinking from the SbQ throughout the nanofibrous mat following photocuring. The SbQ can form intra-fiber bridges, as confirmed by the SEM images; on a macroscopic (gross) scale, the crosslinking manifests itself by the formation of grid-like structures. The thermal properties of the zein nanofibres, however, were minimally improved after the incorporation of SbQ, whereas the cured composite nanofibres demonstrated significantly improved tensile and elongation properties.
Introduction
Electrospinning (ES) is a fundamentally appealing, simple, and versatile technique to produce extremely fine fibrous structures with diameters ranging from 100 nanometres to several micrometres. Electrospun fibers have received significant attention over the last decade because of their nanoscopic dimensions, large surface area-to-volume ratio, and small pore size of the resultant fibres relative to their macroscopic counterparts.1 Not surprisingly, biocompatible electrospun nanofibrous materials have found wide acceptance in the biomedical arena within fields as diverse as tissue engineering scaffolding,2 drug delivery,3 wound dressing,4 and protective clothing.5A universally appealing biocompatible materials that has garnered significant attention recently is zein. Zein, a protein from corn, is available as a by-product of starch processing.6 Zein has received considerable attention due to its non-toxic, biocompatible, biodegradable nature, as well as its excellent film-forming capabilities.7 These latter unique film-forming properties of electrospun zein nanofibres provide a number of host/guest and related applications; for example, they can be used in the encapsulation of essential oils, antioxidants, aromas and flavors in functional food packaging materials,8–10 wound dressings,11 and scaffolding materials for cell/tissue cultures.12 However, the poor mechanical properties of zein have drastically restricted further applications, especially those involving mechanical tolerances; this adversity has usually been addressed by using plasticizers or crosslinking reagents. However, plasticizers such as triethylene glycol have negatively affected the mechanical properties of zein films by contributing to unacceptably enhanced elongation rates without concomitant high strengths and Young's moduli.13 An alternative approach to addressing the films' inherent mechanical handicap is to invoke crosslinking agents. Several of the most frequently invoked crosslinking agents are formaldehyde,14 glyoxal,15 and glutaraldehyde (GDA).16–18 Unfortunately, these crosslinking agents are not as green as desired, while their products suffer from significantly diminished elongation properties. Other relatively green crosslinking reagents have been reported, such as hexamethylenediisocyanate (HDI),19 polycarboxylic acids,20,21 carbodiimide,22 to name a few. Yet, the final products still suffer from either a lack of one or a combination of mechanical properties or environmental friendliness. Herein, a novel atom efficient and two-step crosslinking process is proposed to overcome the inherent lack of desirable physical properties of zein fibrous materials.
SbQ, an amphiphilic sensitizer of the styrylpyridinium family, can be dimerised via an atom economic [2 + 2] orbitally conserved cyclo addition reaction triggered by UV radiation (Scheme 1b).23 SbQ is typically reported along with poly(vinyl alcohol) (PVA) as a covalently grafted pendant group on a polymer backbone.24 In a recent report, the self-assembly (complexation) behavior of SbQ with oppositely charged polyelectrolytes in aqueous solution is an additional coupling technique that was successfully studied as a model system for transporting pharmacologically relevant materials such as paclitaxel (PTX), a mitotic inhibitory agent used extensively as a cancer therapeutic.25 The ability to direct complexed SbQ as a delivery agent for a PTX load demonstrated a promising avenue for delivering hydrophobic chemotherapeutic drugs to tumors.25 However, to date, there is no evidence of studies that have focused on the crosslinking ability of SbQ as a means to selectively couple it to proteins such as zein.
 | ||
| Scheme 1 (a) Self-assembly behavior between zein and SbQ in AcOH solution; and (b) a generic highly atom efficient and conserved photo-dimerization (UV radiation-driven) equation for SbQ. | ||
Therefore, the current research provides for the first time a report on the electrospinning of SbQ from a zein/glacial acetic acid (AcOH) solution into zein/SbQ composite nanofibers that is immediately followed by thermal treatment and UV illumination to induce crosslinking between SbQ and zein. Serendipitously, an incipient self-assembly behavior between zein and SbQ in AcOH solution occurs to further enhance the final chemistry and is illustrated in Scheme 1a; the photochemistry of SbQ is illustrated in Scheme 1b. The morphological, structural, thermal, and mechanical properties of the resulting electrospun zein nanofibers were analyzed before and after crosslinking by SEM, FT-IR, TGA, DSC, and a tensile tester.
Results and discussion
The influence of loaded SbQ on the properties of electrospun solutions
A number of properties for the electrospinning solutions at varying levels of SbQ were measured and are shown in Table 1. The addition of SbQ into the zein/HAc solutions clearly increased the viscosity of the solutions (295 → 495 mPa s), whereas the solution conductivity markedly dropped with increasing SbQ, from 92 μS cm−1 for pure zein/AcOH solution to 64 μS cm−1 and 19 μS cm−1 for Zein90/SbQ10/AcOH and Zein80/SbQ20/AcOH solutions, respectively. Additionally, the surface tension did not significantly change with the addition of SbQ. The increase in viscosity and decrease in conductivity were two significant factors that contributed a great extent to the gradual increase in fibre diameter.| Samples | Viscosity (mPa s) | Conductivity (μS cm−1) | Surface tension (mN m−1) | Average diameter (nm) |
|---|---|---|---|---|
| Zein | 295 ± 7.1 | 92.0 ± 1.9 | 33.1 ± 1.1 | 261.4 ± 98.6 |
| Zein90/SbQ10 | 417.5 ± 3.5 | 64.6 ± 2.3 | 33.0 ± 0.3 | 395.3 ± 89.6 |
| Zein80/SbQ20 | 495 ± 7.1 | 19.4 ± 0.8 | 32.4 ± 0.7 | 451.4 ± 89.7 |
The morphology of the as-spun zein and zein/SbQ nanofibres with different levels of SbQ
Representative SEM images of the electrospun nanofibres are shown in Fig. 1. The pure zein nanofibres demonstrated a beaded structure (Fig. 1a), whereas in a more magnified image, the branched structure can also be seen (Fig. 1a′). After the addition of SbQ, the electrospinnability of zein solutions improved and bead-free individual nanofibres were obtained (Fig. 1b & b′ and c & c′). Moreover, we discovered that the average fibre diameter increased upon the addition of SbQ. The average diameters for the SbQ-loaded fibres increased from 261 nm to 395 nm and 451 nm for zein90/SbQ10 and zein80/SbQ20, respectively. The SbQ content increased the viscosity of the solutions due to increased molecular entanglements in the solution. With respect to conductivity, the addition of SbQ decreased the solution conductivity because of an electrostatic adsorption effect between SbQ and ionized AcOH, as shown in Scheme 1a. Thus, it can be inferred that addition of SbQ resulted in increased fibre diameter due a more compact (less “stretched”) electrified Taylor Cone plume.26The morphology of the zein/SbQ composite nanofibres after thermal or photocuring
Pristine zein nanofibrous membranes are easily shrunk in water and have poor mechanical strength. Therefore, the crosslinking of zein nanofibres is a necessary protocol for any pursuant mechanical gains. For the crosslinking treatment, zein80/SbQ20 was selected as the prototypical sample because it displayed the best fibre morphology.After different post-treatment processes, the zein/SbQ composite nanofibres showed distinct morphologies (Fig. 2). After the thermal treatment, the composite nanofibres showed a very similar structure to that of zein/SbQ nanofibres, differing only in their relatively smaller diameter of 338 nm compared with the original 451 nm (Fig. 2a). However, after UV illumination, the average diameter of the fibres significantly decreased to ∼274 nm, which provides an evidence for the incidence of photo-crosslinking within the fibrous network (Fig. 2b). The diameter of the final zein/SbQ composite nanofibres obtained from a combined thermal and UV illumination treatment was not surprisingly different from that of the other two samples; it may be thus inferred that the thermal treatment may contribute to a more stable structure that resists diameter shrinkage under UV-induced crosslinking (Fig. 2c). Moreover, the fibre morphology changed from cylindrical to fused after the combined treatment. Similar results have also been reported when zein/chitosan/PVP electrospun fibres were crosslinked in a tetrahydrofuran (THF) solution containing 1 wt% HDI.27
 | ||
| Fig. 2 SEM images of zein80/SbQ20 after different post treatment methods: (a) thermal treatment; (b) UV illumination; (c) thermal treatment + UV illumination. | ||
Interaction between zein and SbQ
The ATR-FT-IR spectra over 650–1800 cm−1 are shown in Fig. 3. The zein/SbQ composite nanofibrous membrane containing 20 wt% SbQ was again chosen as the representative sample because of its morphology. Pure zein nanofibres have three characteristic vibrational bands: amide 1 (1644 cm−1), amide 2 (1535 cm−1) and amide 3 (1239 cm−1), which correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, C–N stretching, and N–H in-plane deformation, respectively.17 Upon addition of SbQ, the secondary structure of zein changed, whereas most of the chemical groups of SbQ were well maintained and clearly present in the spectra of zein/SbQ, except that the band at 1695 cm−1 (–CHO group of SbQ) completely disappeared.
O stretching, C–N stretching, and N–H in-plane deformation, respectively.17 Upon addition of SbQ, the secondary structure of zein changed, whereas most of the chemical groups of SbQ were well maintained and clearly present in the spectra of zein/SbQ, except that the band at 1695 cm−1 (–CHO group of SbQ) completely disappeared.
As previously alluded, one of the predominant reaction products of protein –CHO groups with reagents such as glutaraldehyde (GDA), glyoxal, and formaldehyde is a conjugative Schiff base (e.g., a condensation product connected to an ε-amino group of lysine).28 However, in this case, zein lacks lysine, but instead possesses three sulfhydryl groups;29 the only other nucleophilic functional groups that may be available for reaction with the SbQ–CHO group are the N-terminal α-NH2 groups, the imidazole ring of histidine, and the phenolic group of tyrosine.30 Fortunately, in this case, in addition to ethanol and acetone, acetic acid has been reported to have the requisite catalytic properties for protein crosslinking.17
After a period of reaction in acetic acid, the –CHO group of SbQ disappeared and the amide 3 absorption band of zein shifted from 1239 cm−1 to 1247 cm−1, indicative of a chemical reaction between SbQ and zein.
Thermal behaviour of zein/SbQ composite nanofibres
The DSC thermograms of the control and zein/SbQ composite nanofibers following different post-treatments are shown in Fig. 4. From the first scan of the DSC thermograms (Fig. 4a), the different post-treatments lead to very different thermal behaviours. For pure zein fabric, a broad endothermic peak having a peak maximum at ∼117 °C was observed, which is exactly the boiling point of acetic acid. After the addition of SbQ, a sharp peak at ∼140 °C was observed that may be attributed to the conjugate base of acetic acid coulombically bound to the positively charged SbQ. An electrostatic interaction would easily make bound acetic acid more difficult to evaporate, hence, the higher temperature. After thermal treatment (120 °C, 2 hours), the acetic acid disappeared. The peak maximum shifted to ∼100 °C, indicating that the samples contained water.The second scan of the DSC thermograms (Fig. 4b) clearly showed the glass transition temperature (Tg) of all samples. The addition of SbQ did not have a significant effect on augmenting the Tg value, but UV illumination, especially in tandem with thermal treatment, improved the Tg from 164 °C to 167 °C and to 170 °C in conjunction with thermal treatment. An increase in Tg is likely due to restricted chain mobility in zein because of the photo-crosslinked SbQ moieties.
TGA was used to evaluate and compare the thermal stabilities of the zein/SbQ composite nanofibres after different post-treatments. As shown in Fig. 5, zein and zein/SbQ composite nanofibres with different post-treatments showed a mass loss from 50 to 250 °C, which may be attributed to acetic acid or water entrapped within the film matrix. The thermogram of SbQ showed two stages of degradation; the second stage was in the same temperature regime as that of zein. From the thermograms of all these samples, it can be concluded that the incorporation of SbQ did not appreciably change the thermal stability of the zein nanofibres.
 | ||
| Fig. 5 TGA and DTGA thermograms of SbQ, Zein nanofibers, Zein/SbQ composite nanofibers before and after post treatment; the residual weight ratio at 500 °C is presented in the graph. | ||
Water absorption behaviour of zein/SbQ composite nanofibres
Fig. 6(a) displays the representative water absorption curves of zein and zein/SbQ before and after post-treatments. A schematic of the water absorption curve is also shown in Fig. 6(b). The induced weight changes were recorded by the precision electronic balance during the water absorption measurements, as well as when the clamp approached and retracted from the deionized water. The entire process provided a rich source of data on the surface properties and the dynamic wetting behaviour of the samples.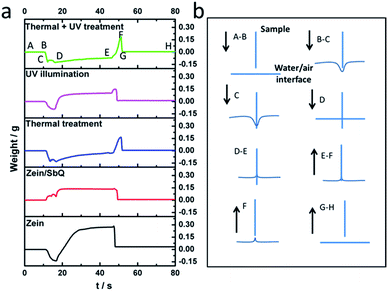 | ||
| Fig. 6 (a) Water absorption behaviour of zein, zein/SbQ before and after post-treatments, (b) schematic illustration of the dynamic wetting process of nanofibre membranes. | ||
The curve started at point A where the weight was zeroed out. When the sample moved towards water, the sample weight was still zero until it was in contact with water. A negative weight change can be observed when the sample is hydrophobic, and vice versa if the sample is hydrophilic. In the case of hydrophobic samples, the surface tension of the water/air interface resists interruption, a factor that contributes to a negative force input from point B to point C. The change between point C and point D is due to a sudden break of the water film when it wicks up around the surface of the sample that was already in the water. After the sample reached its wetting length (5 cm), it was kept static for 30 seconds to adsorb water. Point D to point E represents the weight increase from water absorption after 30 seconds. As the sample started to retract from point E, the water film formed on both sides of the sample surface that stopped it from retracting, and this adhesive force contributed to an obvious increase in weight. At point F, the water film broke, and the weight assumed a value equal to the quantity of water adsorbed.
According to the analysis above, zein behaves as a hydrophobic substrate because of the negative force curve from 10–20 seconds. After ∼20 seconds, the water surface tension is suddenly interrupted, and the substrate can absorb water very rapidly and achieve equilibrium within 10 seconds (at 30 seconds, the water absorption curve plateaus). In general, the adhesive force between water and the sample surface is not appreciable. However, after the incorporation of SbQ, the surface of the sample is far more hydrophilic, as evidenced by the positive force curve in Fig. 6a over the same range as zein. This finding confirmed the speculation that SbQ mainly distributes itself on the surface of the fibre and thus can greatly affect the surface properties. As opposed to the 30 seconds required for zein, zein/SbQ achieved its absorption equilibrium within an order of magnitude less time, i.e., 3 seconds, but showed a very small adhesive force between the sample surface and water (twice as small as observed for zein). This phenomenon could be partially attributed to the surface-bound AcOH in zein, which could form a water-friendly interface. The post-treatments also showed a very significant effect on the dynamic water absorption behaviour. After thermal treatment, the sample became hydrophobic, a finding that might be attributed to the removal of the bound AcOH during the thermal process that forces zein to act as the sole contributor to the surface properties. In addition, the changes in the secondary structure of zein induced by thermal treatment may have lead to increased surface hydrophobicity. Moreover, the UV illumination induced photocrosslinking, a phenomenon that caused SbQ to become much more hydrophobic;24,25 therefore, the sample surface responded in kind. Yet, the dynamic water absorption behaviour was similar to the zein/SbQ nanofibrous membrane, indicative of the presence of AcOH. The Zein/SbQ nanofibrous membrane, after a combined post-treatment, displayed hydrophobic behaviour, a reduced water absorption rate, and a high adhesive force at the water interface.
Mechanical properties
The mechanical properties of electrospun zein fabric samples were tested to determine whether they were altered by the post-treatments. Samples were cut in a parallel fashion to the take-up roll direction. As shown in the stress/strain curves in Fig. 7, the solution-crosslinked zein materials undergo brittle failure as opposed to irregular breakage before failing for the control fabric. Similar results were observed previously with GDA- and formaldehyde-crosslinked zein fabrics, a result that was attributed to improved fiber adhesion.14,18 However, the two-step crosslinked zein fabrics showed very different behaviour during the tensile test. When the applied strength was further increased, the material showed a uniform cross sectional deformation because of the junction points that were present throughout the fiber matrix, and the membrane reached its low yield point before reaching its ultimate tensile strength. This two-step crosslinking process increased both the tensile strength (TS) and elongation, which might be attributed to the different fibrous structures after photo-induced crosslinking. The membrane showed a different shape: a grid-like structure was adopted after photo-crosslinking that is able to resist the applied force by distributing the stress more evenly. The combined juncture points were very strong and were major contributors to significantly improved mechanical properties. | ||
| Fig. 7 Stress/strain curves for electrospun zein nanofibrous membranes before and after crosslinking. | ||
Conclusions
A novel two-step crosslinking process is introduced for the first time that coupled a facile electrospinning process with atom efficient thermal and photocuring treatments to successfully synthesize crosslinked zein composite nanofibrous membranes. The incorporation of SbQ contributed to a bead-free structure that also increased the average diameter of the zein nanofibers. After the two-step crosslinking, the nanofibrous membrane displayed a grid-like structure, which significantly improved the mechanical properties of the crosslinked zein nanofibrous membrane. The aldehyde of SbQ was associated with zein in AcOH solution; after the electrospinning process, SbQ was distributed throughout the as-spun zein fibers. This determination was confirmed by SEM, FTIR, and water absorption properties. The type of crosslinked zein nanofibrous membranes presented herein demonstrated very high mechanical properties that enhance its value for food packaging, scaffolding materials, and numerous bio-product applications.Experimental section
Materials
1-Methyl-4-[2-(4-formylphenyl)-ethenyl]-pyridinium-methosulphate (SbQ) was received from Shanghai Guangyi Printing Equipment Technology Co Ltd (Shanghai, China) and used as received. Zein (Mw = 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was purchased from Sigma-Aldrich (Shanghai, China). Acetic acid was received from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). All materials were used as received without further purification.
000 g mol−1) was purchased from Sigma-Aldrich (Shanghai, China). Acetic acid was received from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). All materials were used as received without further purification.
Materials preparation
The solutions were placed in a syringe with a blunt-end stainless steel needle. The applied voltage was 25 kV with a working distance of 15 cm from the stainless steel needle tip to a collector site (circular rotating drum), and the flow rate was maintained at 1 mL h−1. The nanofibres were collected on the circular rotating drum that was covered with aluminium foil. The compositional characteristics of the as-spun nanofibrous membranes were demarcated as zein (control), zein90/SbQ10 (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 zein
10 zein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SbQ, wt%), zein80/SbQ20 (80
SbQ, wt%), zein80/SbQ20 (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 zein
20 zein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SbQ, wt%).
SbQ, wt%).
Measurements and characterization
Acknowledgements
This research was financially supported by the National High-tech R & D Program of China (2012AA030313), the Changjiang Scholars and Innovative Research Team in University (IRT1135), and the National Natural Science Foundation of China (51006046, 51203064, 21201083 and 51163014). Q. Q. Wang. and A. G. Nandgaonkar contributed equally in carrying out the experiments, analyzing the results, and writing the manuscript.Notes and references
- T. Subbiah, G. Bhat, R. Tock, S. Parameswaran and S. Ramkumar, J. Appl. Polym. Sci., 2005, 96, 557–569 CrossRef CAS.
- S. H. Lim and H. Mao, Adv. Drug Delivery Rev., 2009, 61, 1084–1096, DOI:10.1016/j.addr.2009.07.011.
- E. Kenawy, F. I. Abdel-Hay, M. H. El-Newehy and G. E. Wnek, Mater. Sci. Eng., A, 2007, 459, 390–396, DOI:10.1016/j.msea.2007.01.039.
- J. Chen, G. Chang and J. Chen, Colloids Surf., A, 2008, 313–314, 183–188, DOI:10.1016/j.colsurfa.2007.04.129.
- P. Gibson, H. Schreuder-Gibson and D. Rivin, Colloids Surf., A, 2001, 187–188, 469–481, DOI:10.1016/s0927-7757(01)00616-1.
- Y. Wang and L. Chen, Macromol. Mater. Eng., 2012, 297, 902–913 CrossRef CAS.
- F. Kayaci and T. Uyar, Carbohydr. Polym., 2012, 90, 558–568 CrossRef CAS PubMed.
- D. Alkan, L. Y. Aydemir, I. Arcan, H. Yavuzdurmaz, H. I. Atabay, C. Ceylan and A. Yemenicioğlu, J. Agric. Food Chem., 2011, 59, 11003–11010 CrossRef CAS PubMed.
- K. Shi, J. L. Kokini and Q. Huang, J. Agric. Food Chem., 2009, 57, 2186–2192 CrossRef CAS PubMed.
- Y. P. Neo, S. Ray, J. Jin, M. Gizdavic-Nikolaidis, M. K. Nieuwoudt, D. Liu and S. Y. Quek, Food Chem., 2013, 136, 1013–1021 CrossRef CAS PubMed.
- A. R. Unnithan, G. Gnanasekaran, Y. Sathishkumar, Y. S. Lee and C. S. Kim, Carbohydr. Polym., 2014, 102, 884–892 CrossRef CAS PubMed.
- S. Gong, H. Wang, Q. Sun, S. Xue and J. Wang, Biomaterials, 2006, 27, 3793–3799 CrossRef CAS PubMed.
- G. W. Selling, D. J. Sessa and D. E. Palmquist, Polymer, 2004, 45, 4249–4255 CrossRef CAS PubMed.
- G. W. Selling, K. K. Woods and A. Biswas, Polym. Int., 2011, 60, 537–542 CrossRef CAS.
- G. W. Selling, K. K. Woods and A. Biswas, J. Appl. Polym. Sci., 2012, 123, 2651–2661 CrossRef CAS.
- D. J. Sessa, A. Mohamed, J. A. Byars, S. A. Hamaker and G. W. Selling, J. Appl. Polym. Sci., 2007, 105, 2877–2883 CrossRef CAS.
- D. J. Sessa, A. Mohamed and J. A. Byars, J. Agric. Food Chem., 2008, 56, 7067–7075 CrossRef CAS PubMed.
- G. W. Selling, K. K. Woods, D. Sessa and A. Biswas, Macromol. Chem. Phys., 2008, 209, 1003–1011 CrossRef CAS.
- C. Yao, X. Li and T. Song, J. Appl. Polym. Sci., 2007, 103, 380–385 CrossRef CAS.
- Y. Yang, L. Wang and S. Li, J. Appl. Polym. Sci., 1996, 59, 433–441 CrossRef CAS.
- N. Reddy, Y. Li and Y. Yang, Biotechnol. Prog., 2009, 25, 139–146 CrossRef CAS PubMed.
- S. Kim, D. Sessa and J. Lawton, Ind. Crops Prod., 2004, 20, 291–300 CrossRef CAS PubMed.
- E. S. Cockburn, R. S. Davidson and J. E. Pratt, J. Photochem. Photobiol., A, 1996, 94, 83–88 CrossRef CAS.
- J. Xu, H. Bai, C. Yi, J. Luo, C. Yang, W. Xia and X. Liu, Carbohydr. Polym., 2011, 86, 678–683 CrossRef CAS PubMed.
- Y. Tao, J. Xu, M. Chen, H. Bai and X. Liu, Carbohydr. Polym., 2012, 88, 118–124 CrossRef CAS PubMed.
- Y. P. Neo, S. Ray, A. J. Easteal, M. G. Nikolaidis and S. Y. Quek, J. Food Eng., 2012, 109, 645–651 CrossRef CAS PubMed.
- T. Song and C. Yao, Chin. J. Polym. Sci., 2010, 28, 171–179 CrossRef CAS.
- K. Peters and F. M. Richards, Annu. Rev. Biochem., 1977, 46, 523–551 CrossRef CAS PubMed.
- V. Cabra, R. Arreguin, A. Galvez, M. Quirasco, R. Vazquez-duhalt and A. Farres, J. Agric. Food Chem., 2005, 53, 725–729 CrossRef CAS PubMed.
- A. Habeeb and R. Hiramoto, Arch. Biochem. Biophys., 1968, 126, 16–26 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |



