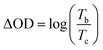Two novel ambipolar donor–acceptor type electrochromic polymers with the realization of RGB (red-green-blue) display in one polymer†
Hui Zhaoa,
Daidi Tangab,
Jinsheng Zhao*b,
Min Wangb and
Jianmin Dou*b
aCollege of Chemical Engineering, China University of Petroleum (East China), Qingdao, 266580, P. R. China
bShandong Key Laboratory of Chemical Energy Storage and Novel Cell Technology, Liaocheng University, Liaocheng, 252059, P. R. China. E-mail: j.s.zhao@163.com; jmdou@lcu.edu.cn
First published on 6th November 2014
Abstract
Two novel electrochromic monomers, 4,7-bis(4-methoxythiophen-2-yl)-[1,2,5]thiadiazolo[3,4-c]pyridine (MOTTP) and 4,7-bis(4-butoxythiophen-2-yl)-[1,2,5]thiadiazolo[3,4-c]pyridine (BOTTP), were synthesized and electropolymerized to give the corresponding polymers PMOTTP and PBOTTP, respectively. For the investigation of their electrochemical and electrochromic properties, the polymers were characterized using cyclic voltammetry (CV), UV-vis spectroscopy, step profiling, and scanning electron microscopy (SEM). The band gaps of the polymers were calculated based on spectroelectrochemical analysis, and were found to be 0.950 eV and 1.088 eV for PMOTTP and PBOTTP, respectively. Electrochromic investigations showed that PMOTTP and PBOTTP showed similar multichromic behaviors: saturated green color in the neutral state, highly transmissive blue in the oxidized state, and saturated red in the reduced state (red-green-blue, RGB). In addition, both polymers have excellent switching properties with more than 60% optical contrast in the NIR region and about a 0.5 s response time from neutral to oxide. Moreover, via electrochemical and spectral analyses both polymers were proven to be n-type dopable polymers. Hence, both polymers are promising materials to complete RGB electrochromic polymers for commercial applications.
Introduction
Since the discovery of the conductivity of doped polyacetylene,1 study in the field of conducting polymers has attracted more and more scientists to be engaged in it. In recent decades, conjugated polymers have shown great potential for applications in organic electronic devices, such as photovoltaic devices,2 light-emitting diodes (LEDs),3 field effect transistors,4 sensors,5 and electrochromic devices.6–8 Electrochromism is defined as a reversible change in the color or optical density of a substance induced by a change in voltage or electrical potential.9,10 Although the earliest electrochromic devices were mostly based on inorganic oxides, such as those of tungsten, iridium, nickel,11 etc., the use of conjugated polymers as active layers in electrochromic devices has received enormous attention because of their high optical contrasts,12 fast switching times13,14 and processability,15 and the ability to fine-tune their band gaps through structure modification.7 To date, examples of electrochromic devices include smart windows,16 rear-view mirrors3 for cars and electrochromic displays. As a type of important electron-rich heterocycle-based polymer, polythiophene and its derivatives are the most commonly used conjugated polymer materials for electrochromic purposes because of their flexibility towards synthetic modifications.Red, green and blue (RGB) are the three main colors for display technology since all other subtractive colors can be achieved by mixing these three.17 Among the RGB colors, neutral state green polymers with highly transmissive oxidized states are very difficult to obtain. This is because to obtain a neutral green polymer, two absorption bands are necessary, one in the red region of the spectrum, and the other in the blue region, leaving an absorption trough in the green region of the spectrum. Furthermore, the two absorption bands should be controlled with the same applied potential, and disappear simultaneously upon successive oxidation to give a bleached transmissive oxidation state.18 The band gaps of the neutral green to transmissive switching polymers are usually less than 1.2 eV, which can only be achieved by means of a donor–acceptor approach. Donor–acceptor systems lead to a narrower band gap because of resonances that enable a stronger double bond character between the donor and acceptor units.19 Since the report of the first neutral green polymer in 2004 (a soluble bithiophene-thienopyrazine copolymer from the Wudl group20 that had a non-transmissive oxidation state) a few green-to-transmissive polymers have been reported by the Reynolds group,21 the Toppare group,22 the Cihaner group,23 etc. These studies open the way for commercialization of electrochromic display devices. However, in the context of low cost and high performance display devices, the main demand is becoming to achieve multi-colored redox achievable states from a single polymer. To date, the examples of neutral state green polymeric materials have still been very rare,24 and examples with additional multicolor properties are welcome.
For the construction of D–A–D type monomers, ethylenedioxythiophene (EDOT) and thiophene derivatives are usually used as the donor units. Meanwhile, 1,2,3-benzotriazole, quinoxaline,25–27 thieno[3,4-b]pyrazine, 2,1,3-benzothiadiazole (BTD) and benzoselenadiazole (BSE)28 are the most preferred acceptor-type units. The [1,2,5]thiadiazolo[3,4-c]pyridine (PT) heterocycle has been employed as an electron-deficient unit in the synthesis of polymer photovoltaic materials and panchromatic organic sensitizers for dye-sensitized mesoscopic solar cells.29,30 Compared with the BTD unit, PT is a stronger acceptor20 due to the presence of the additional pyridine N-atoms.
The donor–acceptor type polymer (PTBT) synthesized with benzotriazole as the acceptor unit and thiophene as the donor unit displayed all three additive colors (RGB) at different oxidation states, and also showed both n- and p-dopable characteristics.31 The discovery of RGB displaying polymers make it possible for the construction of a single component electrochromic display device. Very limited progress concerning the synthesis of RGB displaying polymers has been made since the discovery of PTBT. By employing 3-alkoxy thiophene as the donor unit and PT as the acceptor unit, we report here the synthesis and electrochemical properties of two newly designed donor–acceptor type electrochromic polymers, poly[4,7-bis(4-methoxythiophen-2-yl)-[1,2,5]thiadiazolo[3,4-c]pyridine] (PMOTTP) and poly[4,7-bis(4-butoxythiophen-2-yl)-[1,2,5]thiadiazolo[3,4-c]pyridine] (PBOTTP). Both polymers are green in their neutral states, highly transmissive blue in their oxidized states, and saturated red in their reduced states. The effects of the length of the alkyl chains in the donor unit on the electrochemical and spectral behaviors of the resulting polymers are also discussed in detail.
Experimental
General
All chemicals unless indicated otherwise were purchased from commercial sources and used without further purification, except for tetrahydrofuran which was distilled over Na/benzophenone prior to use. 2,5-Dibromopyridine-3,4-diamine,32,33 4,7-dibromo-[1,2,5]thiadiazolo[3,4-c]pyridine32,33 and tributylstannane34 compounds were prepared according to the literature methods. 1H NMR and 13C NMR spectroscopy studies were carried out on a Varian AMX 400 spectrometer and the chemical shifts (δ) were given relative to tetramethylsilane as the internal standard. Electrochemical synthesis and experiments were performed in a one-compartment cell with a CHI 760C Electrochemical Analyzer controlled by a computer, employing a platinum wire with a diameter of 0.5 mm as the working electrode, a platinum ring as the counter electrode, and a Ag wire (0.02 V vs. SCE.) as the pseudo-reference electrode. Before and after each experiment, the silver pseudo reference was calibrated versus the ferrocene redox couple and then adjusted to match the SCE reference potential. Electrodeposition was performed using a 0.2 M solution of tetrabutylammonium hexafluorophosphate (TBAPF6) at a scan rate of 100 mV s−1 for 20 cycles. Scanning electron microscopy (SEM) measurements were taken using a Hitachi SU-70 thermionic field emission scanning electron microscope. The thickness and surface roughness of the polymer films were measured on a KLA-Tencor D-100 step profiler. UV-vis-NIR spectra were recorded on a Varian Cary 5000 spectrophotometer connected to a computer. A three-electrode cell assembly was used for spectroelectrochemistry measurements where the working electrode was indium tin oxide (ITO) glass, the counter electrode was a stainless steel wire, and a Ag wire was used as the pseudo reference electrode. The polymer films for spectroelectrochemistry were prepared using potentiostatic deposition on ITO glass slides (the active area: 1.0 cm × 2.8 cm). The thickness of the polymer films grown potentiostatically on ITO was controlled by the total charge passed through the cell and was measured using a step profiler. Digital photographs of the polymer films were taken using a Canon Power Shot A3000 IS digital camera. Elemental analyses are determined using a Thermo Finnigan Flash EA 1112, CHNS-O elemental analysis instrument. Mass spectrometry analysis was conducted using a Bruker maXis UHR-TOF mass spectrometer.Synthesis procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) gave 3,4-diamino-2,5-dibrompyridine as a white flocculent material (yield = 51%). Mp: 216–218 °C. 1H NMR (400 MHz, DMSO, δ/ppm): 7.53 (s, 1H, pyridic-H), 5.99 (s, 2H, N–H), 5.03 (s, 2H, N–H). 13C NMR (100 MHz, CDCl3, δ/ppm): 139.93, 139.13, 129.54, 126.67, 106.22. MS (C5N3Br2H5) m/z: calcd for 266.9; found 266.8. Anal. calcd for (C5N3Br2H5): C, 22.5; N, 15.7; Br, 59.9; H, 1.9. Found: C, 22.4; N, 15.7; Br, 60.0, H, 1.9.
1) gave 3,4-diamino-2,5-dibrompyridine as a white flocculent material (yield = 51%). Mp: 216–218 °C. 1H NMR (400 MHz, DMSO, δ/ppm): 7.53 (s, 1H, pyridic-H), 5.99 (s, 2H, N–H), 5.03 (s, 2H, N–H). 13C NMR (100 MHz, CDCl3, δ/ppm): 139.93, 139.13, 129.54, 126.67, 106.22. MS (C5N3Br2H5) m/z: calcd for 266.9; found 266.8. Anal. calcd for (C5N3Br2H5): C, 22.5; N, 15.7; Br, 59.9; H, 1.9. Found: C, 22.4; N, 15.7; Br, 60.0, H, 1.9.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to give a yellow solid. Mp: 115 °C. 1H NMR (300 MHz, CDCl3, δ/ppm): 8.552 (s, 1H, pyridic-H). 13C NMR (100 MHz, CDCl3, δ/ppm): 162.79, 160.88, 153.17, 139.18, 96.22. MS (C5SN3Br2H) m/z: calcd for 294.9; found 294.7. Anal. calcd for (C5SN3Br2H): C, 20.4; S, 10.9; N, 14.2; Br, 54.2; H, 0.3. Found: C, 20.3; S, 10.8; N, 14.3; Br, 54.3; H, 0.3.
1, v/v) to give a yellow solid. Mp: 115 °C. 1H NMR (300 MHz, CDCl3, δ/ppm): 8.552 (s, 1H, pyridic-H). 13C NMR (100 MHz, CDCl3, δ/ppm): 162.79, 160.88, 153.17, 139.18, 96.22. MS (C5SN3Br2H) m/z: calcd for 294.9; found 294.7. Anal. calcd for (C5SN3Br2H): C, 20.4; S, 10.9; N, 14.2; Br, 54.2; H, 0.3. Found: C, 20.3; S, 10.8; N, 14.3; Br, 54.3; H, 0.3.General procedure for the synthesis of MOTTP and BOTTP via Stille coupling
4,7-Dibromo-[1,2,5]thiadiazolo[3,4-c]pyridine (1.00 g, 3.4 mmol) and an excess of tributyl(3-methoxythiophen-2-yl)stannane (17 mmol) or tributyl(3-butoxythiophen-2-yl)stannane (17 mmol) with Pd(PPh3)2Cl2 (0.238 g, 0.34 mmol) as the catalyst were dissolved in anhydrous toluene (80 ml) at room temperature. The solution was stirred under a nitrogen atmosphere for 30 min. The temperature was raised immediately until the solution was at reflux. The mixture was stirred under a nitrogen atmosphere for 24 h, cooled and concentrated on a rotary evaporator. Finally, the residue was purified by column chromatography on silica gel using hexane–dichloromethane as the eluent.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) to give MOTTP as a red solid (0.8 g, 67%). Mp: 186 °C. 1H NMR (400 MHz, CDCl3, δ/ppm): 8.77 (s, 1H, pyridic-H), 8.34 (d, 1H, thienyl-H), 7.77 (d, 1H, thienyl-H), 6.56 (d, 1H, thienyl-H), 6.42 (d, 1H, thienyl-H), 3.89 (d, 6H, –OCH3). 13C NMR (100 MHz, CDCl3, δ/ppm): 159.55, 159.17, 146.28, 140.50, 135.27, 123.34, 120.02, 103.19, 99.27, 57.56. MS (C13H11N3S3O2) m/z: calcd for 337.4; found 337.5. Anal. calcd for (C13H11N3S3O2): C, 46.3; N, 12.4; O, 9.5; S, 28.5; H, 3.3. Found: C, 46.5; N, 12.4; O, 9.4; S, 28.3; H, 3.4.
2, v/v) to give MOTTP as a red solid (0.8 g, 67%). Mp: 186 °C. 1H NMR (400 MHz, CDCl3, δ/ppm): 8.77 (s, 1H, pyridic-H), 8.34 (d, 1H, thienyl-H), 7.77 (d, 1H, thienyl-H), 6.56 (d, 1H, thienyl-H), 6.42 (d, 1H, thienyl-H), 3.89 (d, 6H, –OCH3). 13C NMR (100 MHz, CDCl3, δ/ppm): 159.55, 159.17, 146.28, 140.50, 135.27, 123.34, 120.02, 103.19, 99.27, 57.56. MS (C13H11N3S3O2) m/z: calcd for 337.4; found 337.5. Anal. calcd for (C13H11N3S3O2): C, 46.3; N, 12.4; O, 9.5; S, 28.5; H, 3.3. Found: C, 46.5; N, 12.4; O, 9.4; S, 28.3; H, 3.4.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) to give BOTTP as a red solid (0.76 g, 50%). Mp: 149 °C. 1H NMR (400 MHz, CDCl3, δ/ppm): 8.78 (s, 1H, pyridic-H), 8.38 (d, 1H, thienyl-H), 7.78 (d, 1H, thienyl-H), 6.55 (d, 1H, thienyl-H), 6.41 (d, 1H, thienyl-H), 4.03 (m, 4H, –OCH2), 1.81 (m, 4H, –CH2), 1.33 (m, 4H, –CH2), 0.99 (t, 6H, –CH3). 13C NMR (100 MHz, CDCl3, δ/ppm): 158.47, 154.97, 146.34, 139.66, 135.01, 123.89, 120.29, 103.62, 99.61, 70.22, 31.53, 19.47, 14.05. MS (C13H23N3S3O2) m/z: calcd for 349.5; found 349.4. Anal. calcd for (C13H23N3S3O2): C, 44.7; N, 12.0; O, 9.2; S, 27.5; H, 6.6. Found: C, 44.5; N, 12.1; O, 9.2; S, 27.7; H, 6.5.
3, v/v) to give BOTTP as a red solid (0.76 g, 50%). Mp: 149 °C. 1H NMR (400 MHz, CDCl3, δ/ppm): 8.78 (s, 1H, pyridic-H), 8.38 (d, 1H, thienyl-H), 7.78 (d, 1H, thienyl-H), 6.55 (d, 1H, thienyl-H), 6.41 (d, 1H, thienyl-H), 4.03 (m, 4H, –OCH2), 1.81 (m, 4H, –CH2), 1.33 (m, 4H, –CH2), 0.99 (t, 6H, –CH3). 13C NMR (100 MHz, CDCl3, δ/ppm): 158.47, 154.97, 146.34, 139.66, 135.01, 123.89, 120.29, 103.62, 99.61, 70.22, 31.53, 19.47, 14.05. MS (C13H23N3S3O2) m/z: calcd for 349.5; found 349.4. Anal. calcd for (C13H23N3S3O2): C, 44.7; N, 12.0; O, 9.2; S, 27.5; H, 6.6. Found: C, 44.5; N, 12.1; O, 9.2; S, 27.7; H, 6.5.Results and discussion
Synthesis of monomers
The synthesis of MOTTP and BOTTP was carried out with slight modifications of the well-established literature procedures (Scheme 1). The first step of this route involved the bromination of pyridine-3,4-diamine in the presence of an HBr/Br2 mixture. In the second step, 4,7-dibromo-[1,2,5]thiadiazolo[3,4-c]pyridine was obtained through oxidation with an excess amount of SOCl2 in pyridine as the solvent. The organotin compound was synthesized following previous literature.35 In the last step, the Stille coupling reaction was achieved in anhydrous toluene in the presence of a catalytic amount of Pd(PPh3)2Cl2. The reactions proceeded quite nicely to afford MOTTP and BOTTP in satisfactory yields.Electrochemical polymerization
The electrochemical properties of the monomers and their polymers were examined using cyclic voltammetry. Both polymers were deposited on Pt wire using cyclic voltammetry with a single scan rate (100 mV s−1) in an acetonitrile (ACN)–dichloromethane (DCM) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by volume) solvent mixture containing 0.2 M tetrabutylammonium hexafluorophosphate (TBAPF6) as the supporting electrolyte and 0.005 M of the monomers. Voltammetry curves for the repeated scanning electropolymerization of MOTTP and BOTTP are shown in Fig. 1. The first cycle of the CV test corresponded to oxidation of the monomer and the onset oxidation potentials (Eonset) of MOTTP and BOTTP are 1.06 and 1.07 V, respectively. During the repetitive anodic potential scan, the generation of a new quasi-reversible redox couple (Eoxp = 0.77 V, Eredp = 0.62 V for MOTTP and Eoxp = 0.81, Eredp = 0.82 V for BOTTP) and the increase in the current intensity of this quasi-reversible redox peak indicated the formation of highly electroactive polymers on the surface of the working electrodes. The oxidation potential of MOTTP was lower than that of BOTTP since the electron donating effect of BOTTP decreased due to the sterically bulky groups introduced by the butyl chains , resulting in a less efficient overlap between the π-orbital of the oxygen atom and the conjugated system. It can thus be concluded that the redox reaction is highly sensitive to the steric and electronic effects of the substituent.35
1, by volume) solvent mixture containing 0.2 M tetrabutylammonium hexafluorophosphate (TBAPF6) as the supporting electrolyte and 0.005 M of the monomers. Voltammetry curves for the repeated scanning electropolymerization of MOTTP and BOTTP are shown in Fig. 1. The first cycle of the CV test corresponded to oxidation of the monomer and the onset oxidation potentials (Eonset) of MOTTP and BOTTP are 1.06 and 1.07 V, respectively. During the repetitive anodic potential scan, the generation of a new quasi-reversible redox couple (Eoxp = 0.77 V, Eredp = 0.62 V for MOTTP and Eoxp = 0.81, Eredp = 0.82 V for BOTTP) and the increase in the current intensity of this quasi-reversible redox peak indicated the formation of highly electroactive polymers on the surface of the working electrodes. The oxidation potential of MOTTP was lower than that of BOTTP since the electron donating effect of BOTTP decreased due to the sterically bulky groups introduced by the butyl chains , resulting in a less efficient overlap between the π-orbital of the oxygen atom and the conjugated system. It can thus be concluded that the redox reaction is highly sensitive to the steric and electronic effects of the substituent.35
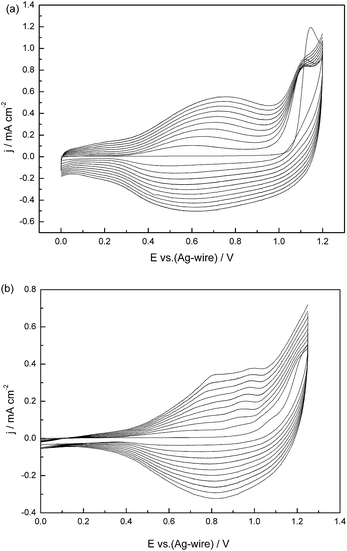 | ||
Fig. 1 Cyclic voltammogram curves of MOTTP (a) and BOTTP (b) in ACN–DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing 0.2 M TBAPF6 at a scan rate of 100 mV s−1. 1) containing 0.2 M TBAPF6 at a scan rate of 100 mV s−1. | ||
The electrochemical behaviors of the polymer films (prepared on platinum wires by sweeping the potentials for three cycles) were characterized using CV at different scan rates between 25 and 300 mV s−1 in monomer-free electrolyte solution. The CV curves of PMOTTP and PBOTTP at different scan rates between 25 and 300 mV s−1 during the p-doping process are shown in Fig. 2a and b, respectively. A couple of redox peaks with an oxidation potential of 0.79 V and a reduction potential of 0.74 V were clearly observed during the p-doping process for PMOTTP. A couple of redox peaks for PBOTTP were located at 0.91 and 0.80 V. The scan rate dependence of the peak current density is illustrated in Fig. 2c. As seen in Fig. 2c, the peak current densities show a linear relationship as a function of scan rate for both polymers, which indicates that the electrochemical processes are not diffusion limited and are quasi-reversible even at high scan rates.8 The CV curves of PMOTTP and PBOTTP at a scan rate of 100 mV s−1 during the n-doping and p-doping processes are shown in Fig. 2d. A quasi-reversible p-style doping and a quasi-reversible n-style doping can be seen. In the reduction region, a sharp redox peak (Eoxp = −0.87 V, Eredp = −0.78 V for PMOTTP and Eoxp = −0.88, Eredp = −0.79 V for PBOTTP) is observed which indicates that the polymer has an n-dopable character. Furthermore, it is notable that the redox peaks of the n-doping/dedoping were much stronger than those of the p-doping/dedoping process. The results indicate that [1,2,5]thiadiazolo[3,4-c]pyridine (PT) is a strong electron acceptor and PMOTTP and PBOTTP are good n-type conjugated polymers. As clearly stated in literature,36 negatively doped waves in CV do not necessarily mean that a true n-type doping process is available. Hence, additional support must be given via spectroelectrochemistry studies.
The stability and robustness of the polymer films are quite important parameters for them to be amenable for use in numerous practical applications. The stability of each polymer film without purging the electrolyte solution with an inert gas was investigated in monomer-free electrolyte solution by applying potential pulses. The polymer films were prepared on Pt wires by sweeping the potentials for five cycles at 100 mV s−1 in an acetonitrile (ACN)–dichloromethane (DCM) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by volume) solvent mixture containing 0.2 M TBAPF6 and 0.005 M of the monomers, and their cyclic voltammograms were recorded with 1000 cycles at 200 mV s−1 in monomer-free electrolyte solution. The total charge involved during the electrochemical process was calculated for each voltammogram cycle. Fig. 3 shows the change in the CV curves for the PMOTTP and PBOTTP films between the 1st and 1000th cycles. As depicted in Fig. 3a, the loss of the total charge of PMOTTP was approximately 10% between the initial and 1000th cycles. For PBOTTP, the loss of the total charge was approximately 11% between the initial and 1000th cycles (see Fig. 3b). The stability test was carried out without the exclusion of air in order to test the environmental stability of the polymers, since the presence of air has been supposed to have a detrimental effect on the stability of materials.12 We suspect that the observed loss is most probably due to the degradation reactions catalyzed by the presence of oxygen and trace water in the environment. On the other hand, the electropolymerization procedure under environmental conditions may lead to the formation of oligomers, which impedes good film-forming properties and the adhesion to the substrate.
1, by volume) solvent mixture containing 0.2 M TBAPF6 and 0.005 M of the monomers, and their cyclic voltammograms were recorded with 1000 cycles at 200 mV s−1 in monomer-free electrolyte solution. The total charge involved during the electrochemical process was calculated for each voltammogram cycle. Fig. 3 shows the change in the CV curves for the PMOTTP and PBOTTP films between the 1st and 1000th cycles. As depicted in Fig. 3a, the loss of the total charge of PMOTTP was approximately 10% between the initial and 1000th cycles. For PBOTTP, the loss of the total charge was approximately 11% between the initial and 1000th cycles (see Fig. 3b). The stability test was carried out without the exclusion of air in order to test the environmental stability of the polymers, since the presence of air has been supposed to have a detrimental effect on the stability of materials.12 We suspect that the observed loss is most probably due to the degradation reactions catalyzed by the presence of oxygen and trace water in the environment. On the other hand, the electropolymerization procedure under environmental conditions may lead to the formation of oligomers, which impedes good film-forming properties and the adhesion to the substrate.
 | ||
Fig. 3 Electrochemical stability of PMOTTP (a) and PBOTTP (b) in monomer-free ACN–DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing 0.2 M TBAPF6 after 1000 switching cycles by CV. 1) containing 0.2 M TBAPF6 after 1000 switching cycles by CV. | ||
Morphology and thickness
The surface morphologies of the polymer films were analyzed using SEM. The films were prepared using a constant potential in an ACN–DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by volume) solution containing 0.2 M TBAPF6 and 0.005 M of the relevant monomer on an ITO electrode with the same polymerization charge of 3.0 × 10−2 C and were dedoped before characterization. The SEM images of these polymer films are shown in Fig. 4a. The PMOTTP film exhibits a compact morphology with a stack of granules. The diameters of the granules range from 4 to 24 μm. In contrast, the PBOTTP film exhibits an irregular coral-like porous structure grown with small granules, and the approximate diameters of the granules are in the range of 2–12 μm. These morphologies can facilitate the movement of doping anions into and out of the polymer film during the doping and dedoping processes, in good agreement with the good redox activity of the films.37
1, by volume) solution containing 0.2 M TBAPF6 and 0.005 M of the relevant monomer on an ITO electrode with the same polymerization charge of 3.0 × 10−2 C and were dedoped before characterization. The SEM images of these polymer films are shown in Fig. 4a. The PMOTTP film exhibits a compact morphology with a stack of granules. The diameters of the granules range from 4 to 24 μm. In contrast, the PBOTTP film exhibits an irregular coral-like porous structure grown with small granules, and the approximate diameters of the granules are in the range of 2–12 μm. These morphologies can facilitate the movement of doping anions into and out of the polymer film during the doping and dedoping processes, in good agreement with the good redox activity of the films.37
 | ||
| Fig. 4 SEM images of (a1) PMOTTP and (a2) PBOTTP, and step profiler images of (b1) PMOTTP and (b2) PBOTTP deposited potentiostatically onto ITO electrodes. | ||
The thickness and roughness of the polymer films were investigated using a step profiler. The thickness images are shown in Fig. 4b. As seen from Fig. 4b, the thicknesses of PMOTTP and PBOTTP were 830 nm and 1002 nm, respectively. The images of the step profiler measurements reveal that the polymer films have extremely rough surfaces with detectable pits, which is in good agreement with the morphologies shown in the SEM images. Fig. 4b also reveals that PMOTTP has a lower average roughness value compared to PBOTTP due to the compact morphology of PMOTTP.
Optical properties of the monomers and films
The UV-vis absorption spectra of the monomers dissolved in CH2Cl2 and the corresponding dedoped polymer films deposited on ITO electrodes were examined. The absorption spectra of these novel monomers and films in their neutral states are shown in Fig. 5. As the Fig. 5 shows, both monomers exhibited two characteristic absorption bands as the typical features of donor–acceptor conjugated compounds, which were assigned to the π–π* transition and intramolecular charge transfer, respectively. Two obvious absorption peaks were observed at 308 and 482 nm for MOTTP, and 309 and 487 nm for BOTTP. Meanwhile, the optical band gaps (Eg) of the monomers were precisely calculated from the low energy absorption edges (λonset) (Eg = 1241/λonset). The Eg values of MOTTP and BOTTP based on λonset (λonset = 563 nm for MOTTP, 561 nm for BOTTP) were calculated to be 2.20 eV and 2.21, eV respectively. Compared with MOTTP, BOTTP has a small blue shift in the low energy absorption band and a slightly higher band gap due to the fact that the introduction of a longer butyl chain in place of methyl causes greater steric hindrance, resulting in less order and less conjugation. | ||
| Fig. 5 UV-vis absorption spectra of MOTTP and BOTTP in DCM. Inset: absorption spectra of the corresponding polymers deposited on ITO in their neutral states. | ||
The UV-vis absorption spectra of the neutral-state films are shown in the inset of Fig. 5. The location of the absorption maxima and intensity of the main π–π* peak give some insight into the polymer color. As seen from the inset of Fig. 5, both polymers presented two well-separated maximal absorption peaks in the visible region which were assigned to the strong π–π* transition and intramolecular charge transfer in the neutral state. The absorption peaks were located at 435 nm and 850 nm for PMOTTP and for PBOTTP were located at 432 nm and 848 nm, which are essential values for a neutral green state. A minimum absorption is observed at around 514 nm for PMOTTP, and the difference in the transmittances of the peaks at 435 and 850 nm is excellent for producing a highly saturated green color. PBOTTP exhibited a similar spectroelectrochemistry behavior. The Eg values of PMOTTP and PBOTTP based on λonset (1300 nm for PMOTTP, 1140 nm for PBOTTP) were calculated to be 0.95 eV and 1.088 eV, respectively (see Table 1). As shown in Table 1, PMOTTP has an apparent red shift in the low-energy absorption wavelengths and a lower band gap than PBOTTP. It is suggested that the introduction of the butyl group on the donor unit increased the repulsive steric effect on neighboring repeated units, which finally brought about a decrease in effective conjugation length in the homopolymer and a slightly higher band-gap.35 Moreover, the polymers experienced a red shift in the absorption maxima compared to the corresponding monomers, which is attributable to the inter-chain interaction caused by π-stacking of the polymers. The parameters of 4,7-bis(2,3-dihydrothieno[3,4-b][1,4]dioxin-7-yl)benzo[c][1,2,5]thiadiazole (PBDT)38 are also listed in Table 1 in order to compare it with the newly prepared polymers. In comparison, the optical band gaps of PMOTTP and PBOTTP are somewhat lower than that of PBDT which demonstrated that PMOTTP and PBOTTP were more outstanding D–A type conducting polymers than PBDT.38
| Compounds | Eonset (V) | λmax (nm) | vs. (Ag-wire) λonset (nm) | Ega (eV) | Egd (eV) | HOMOb (eV) | LUMOc (eV) |
|---|---|---|---|---|---|---|---|
| a Calculated from the low energy absorption edges (λonset), Eg = 1241/λonset.b HOMO = −e(Eonset + 4.4) (Eonset vs. SCE).c Calculated by the subtraction of the optical band gap from the HOMO level.d Eg is calculated based on DFT.e Data were taken from ref. 38. | |||||||
| MOTTP | 1.06 | 308![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 482 482 |
563 | 2.20 | 1.956 | −5.48 | −3.28 |
| BOTTP | 1.07 | 309![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 487 |
561 | 2.21 | 2.032 | −5.49 | −3.28 |
| PMOTTP | 0.039 | 435![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
1300 | 0.95 | — | −4.459 | −3.509 |
| PBOTTP | 0.035 | 432![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 848 848 |
1140 | 1.088 | — | −4.455 | −3.367 |
| PBDTe | — | 428![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 755 755 |
1043 | 1.191 | — | — | — |
To obtain the precise electrical band gaps of the monomers, DFT calculations were carried out on the density functional theory (DFT) level, employing the Gaussian 05 program. The ground-state electron density distributions of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) are illustrated in Fig. 6. The values of the HOMO and LUMO are −5.627 and −3.666 for MOTTP, and −5.586 and −3.554 for BOTTP. The band gaps are calculated to be 1.956 and 2.032 for MOTTP and BOTTP respectively. These values were found to be lower than the values from the experimental data. This is mainly due to various effects, such as solvent effects, and to variation in the solid state compared to the gaseous states. However, the band gap of MOTTP based on DFT calculations is slightly lower than that of BOTTP, which is exactly consistent with the band gaps based on optical experiments.
 | ||
| Fig. 6 The ground-state electron density distributions of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). | ||
In view of the device and high-performance display applications, the optical properties of the polymers should be manifested by using the changes in the optical absorption spectra under various voltage pulses. Potentiostatic deposition was performed in an ACN–DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by volume) solution containing 0.2 M TBAPF6 as a supporting electrolyte and 0.005 M of the monomers. The PMOTTP and PBOTTP films were electrodeposited onto ITO with the same polymerization charge of 2.0 × 10−2 C at 1.20 V and 1.25 V, respectively. After polymerization, electrochemical dedoping was carried out at 0 V in monomer-free ACN–DCM (1
1, by volume) solution containing 0.2 M TBAPF6 as a supporting electrolyte and 0.005 M of the monomers. The PMOTTP and PBOTTP films were electrodeposited onto ITO with the same polymerization charge of 2.0 × 10−2 C at 1.20 V and 1.25 V, respectively. After polymerization, electrochemical dedoping was carried out at 0 V in monomer-free ACN–DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
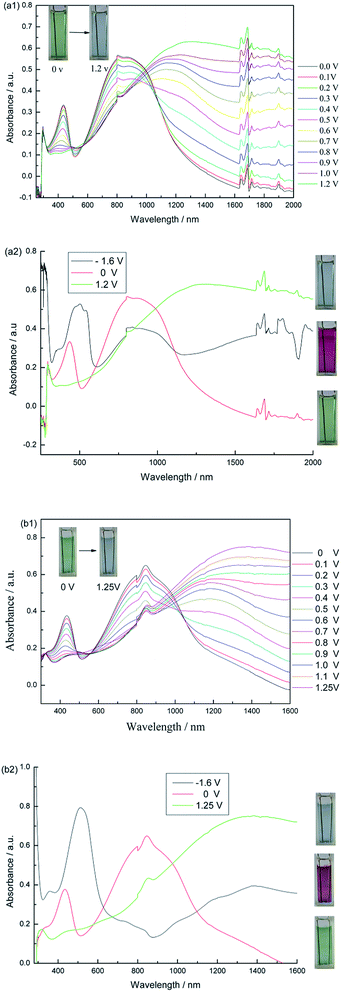
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by volume) containing 0.2 M TBAPF6, and then the in situ electronic absorption spectra of the polymer films upon stepwise oxidation were investigated. Fig. 7 shows the spectroelectrochemistry and corresponding colors of the PMOTTP and PBOTTP films. As seen from Fig. 7, both polymers exhibited two transitions due to their donor–acceptor nature in the neutral state. The two transitions in the D–A type polymers were attributed to the transitions from the thiophene-based valence band to its antibonding counterpart (high-energy transition) and to the substituent-localized conduction band (low energy transition). Hence, interactions between donor and acceptor units (their match) determined the energy and intensity of these transitions.39 The intensities of the low energy transitions for the polymers are apparently higher than those of the corresponding high-energy transitions, as observed from the absorption peak in the visible region.
1, by volume) containing 0.2 M TBAPF6, and then the in situ electronic absorption spectra of the polymer films upon stepwise oxidation were investigated. Fig. 7 shows the spectroelectrochemistry and corresponding colors of the PMOTTP and PBOTTP films. As seen from Fig. 7, both polymers exhibited two transitions due to their donor–acceptor nature in the neutral state. The two transitions in the D–A type polymers were attributed to the transitions from the thiophene-based valence band to its antibonding counterpart (high-energy transition) and to the substituent-localized conduction band (low energy transition). Hence, interactions between donor and acceptor units (their match) determined the energy and intensity of these transitions.39 The intensities of the low energy transitions for the polymers are apparently higher than those of the corresponding high-energy transitions, as observed from the absorption peak in the visible region.
To further elucidate the optoelectronic properties of these thin polymer films, in situ spectroelectrochemical measurements were performed wherein the absorption spectra were measured as increasingly higher oxidizing potentials were applied across the polymer film. As shown in Fig. 7-a1, upon oxidation of the polymer PMOTTP, the intensity of the absorption of the two π–π* transition bands (centered at 435 and 850 nm) started to decrease simultaneously with a concomitant increase in the near-IR region (beyond 1400 nm), indicating the formation of polarons40 and bipolarons.41 In the fully oxidized state of the polymer PMOTTP (1.2 V), the newly formed lower energy electronic transitions lie sufficiently outside the visible region with little tailing from the NIR, which makes the polymer present a highly transmissive blue color in the oxidized states. In this case, PMOTTP can switch between the neutral green color to the highly transmissive blue color in the oxidized state. As shown in Fig. 7-b1, the PBOTTP film experienced a similar trend in change to that of PMOTTP as the potential stepped from 0 V (neutral state) to 1.25 V (oxidized state). The UV-vis spectra for PMOTTP and PBOTTP (Fig. 7) display well-defined isosbestic points at approx. 564 nm and 558 nm, respectively, indicating that the PMOTTP and PBOTTP polymers were being interconverted between two distinct forms on both occasions: the neutral form and radical cation.39
Conducting polymers with stable negatively doped states receive tremendous interest, since a more complicated device structure such as those of LEDs3 and ambipolar field effect transistors can be attained with these materials. In addition to reduction waves in CV, evidence for charge carrier formation upon reduction should also be studied by spectroelectrochemistry. Absorption spectra of the polymers at the reduction potential of −1.6 V were investigated to characterize the optical changes that occurred during the n-doping process and prove the introduction of charge carriers to the conjugated systems in the n-doped state. As shown in Fig. 7, upon the switch from the neutral to the reduced state, several similar changes have been seen for the UV-vis-NIR spectra of the two polymers, including a moderate absorption increase in the NIR region, a weakening or even disappearance of the absorption band at around 850 nm, a red-shift in the π–π* transition band, and the appearance of apparent and well-defined absorption peaks at around 500 nm in the visible region. The maximum absorption peaks were centered at 508 nm and 512 nm for PMOTTP and PBOTTP, respectively, which gives rise to a saturated red color in the reduced states of the polymers. Hence, with the strong absorption changes in the NIR region and the CV waves observed at negative potentials, it is clear that both PMOTTP and PBOTTP are displaying true n-type doping processes.37 It is interesting that these polymers are unique in the literature to date with their highly saturated green color in the neutral state, highly transmissive blue colour in the oxidized state and saturated red colour in the reduced state. To further establish the colors of the polymers, the colorimetric properties were characterized using CIE1976 color space (L*a*b*). The values of the relative luminance (L), hue (a) and saturation (b) were measured in the neutral, oxidized and reduced states of the polymers and are summarized in Table 2.
| Polymers | E, vs. (Ag wire) (V) | L | a | b | Colors |
|---|---|---|---|---|---|
| PMOTTP | 0 | 61 | −9 | 11 | Saturated green |
| 1.2 | 60 | −5 | −4 | Transmissive blue | |
| −1.6 | 42 | 43 | −1 | Red | |
| PBOTTP | 0 | 63 | −17 | 13 | Saturated green |
| 1.25 | 60 | −4 | −1 | Transmissive blue | |
| −1.6 | 43 | 25 | 2 | Red |
Switching properties
The stabilities, optical contrasts, and response times of the polymer films upon electrochromic switching between their neutral and oxidized states have been monitored both in the visible and NIR regions. The studies were performed with a switching interval of 4 s at their dominant wavelengths by multi-potential steps, switching repeatedly between their fully neutral and oxidized states in a monomer-free ACN–DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
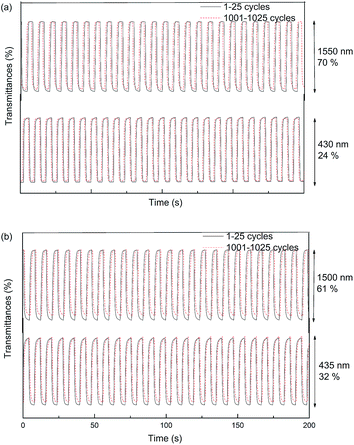
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by volume) solution containing 0.2 M TBAPF6 as a supporting electrolyte. The optical contrasts, response times and coloration efficiencies of PMOTTP, PBOTTP and PBDT based on electrochromic switching at different given wavelengths are shown in Table 3. PBDT was used for comparison with the new polymers. The optical contrast (ΔT%), one of the crucial factors in appraising an electrochromic material, is defined as the percent transmittance change at a specified wavelength between the redox states. Fig. 8 shows the electrochromic switching properties of PMOTTP between 0 and 1.2 V and PBOTTP between 0 and 1.25 V at two different wavelengths in both visible and NIR regions. The optical contrasts of PMOTTP (24% at 430 nm, 70% at 1550 nm) and PBOTTP (32% at 435 nm, 61% at 1500 nm) are satisfactory. Compared with the structural analogue polymer PBDT, the newly prepared polymers have comparable optical contrasts in both the visible and the NIR region. The outstanding optical contrast in the NIR region is a very significant property for many NIR applications. After 1000 cycles of switching, the polymers kept working without significant loss in the percent transmittance contrast value. This indicated that the polymers have quite high optical stabilities, retaining 96% at 430 nm and 97% at 1550 nm for PMOTTP and 94% at 435 nm and 95% at 1500 nm for PBOTTP after 1000 cycles of switching.
1, by volume) solution containing 0.2 M TBAPF6 as a supporting electrolyte. The optical contrasts, response times and coloration efficiencies of PMOTTP, PBOTTP and PBDT based on electrochromic switching at different given wavelengths are shown in Table 3. PBDT was used for comparison with the new polymers. The optical contrast (ΔT%), one of the crucial factors in appraising an electrochromic material, is defined as the percent transmittance change at a specified wavelength between the redox states. Fig. 8 shows the electrochromic switching properties of PMOTTP between 0 and 1.2 V and PBOTTP between 0 and 1.25 V at two different wavelengths in both visible and NIR regions. The optical contrasts of PMOTTP (24% at 430 nm, 70% at 1550 nm) and PBOTTP (32% at 435 nm, 61% at 1500 nm) are satisfactory. Compared with the structural analogue polymer PBDT, the newly prepared polymers have comparable optical contrasts in both the visible and the NIR region. The outstanding optical contrast in the NIR region is a very significant property for many NIR applications. After 1000 cycles of switching, the polymers kept working without significant loss in the percent transmittance contrast value. This indicated that the polymers have quite high optical stabilities, retaining 96% at 430 nm and 97% at 1550 nm for PMOTTP and 94% at 435 nm and 95% at 1500 nm for PBOTTP after 1000 cycles of switching.
Response time, another of the most important characteristics of electrochromic materials, is the necessary time for 95% of the full optical switch to occur (after which the naked eye could not sense the color change).42 PMOTTP has excellent switching times from neutral to oxide of 0.439 s at 430 nm and 0.438 s at 1550 nm. PBOTTP has comparable switching times of 0.510 s at 435 nm and 0.550 s at 1500 nm. In comparison, it can be easily found that the PMOTTP film showed a slightly faster switch time than that of PBOTTP. The faster switching response could be ascribed to the faster dopant ion diffusion during the switch time of the new polymers, which is faster than that of PBDT in NIR region and is comparable in the visible region.
The coloration efficiency (CE) is also an important characteristic for electrochromic materials, which is defined as the relation between the injected/ejected charge as a function of electrode area (ΔQ) and the change in optical density (ΔOD) at a specific dominant wavelength (λmax) as illustrated by the following equation:40
where the Tb is the transmission in the bleached state and Tc is the transmission in the colored state. Tc and Tb values are measured at a nominated wavelength (typically the wavelength producing the maximum optical density change, ΔODλmax = log(Tb/Tc)λmax). ΔQ is the charge density, the charge ingress/egress divided by the geometric electrode area of the polymer. CE is expressed in units of cm2 C−1. The coloration efficiencies of PMOTTP were found to be 76.9 cm2 C−1 at 430 nm and 249 cm2 C−1 at 1550 nm during the p-doping process, whereas the values for PBOTTP were 99 cm2 C−1 at 435 nm and 259 cm2 C−1 at 1500 nm.
Conclusions
Novel D–A type monomers based on [1,2,5]thiadiazolo[3,4-c]pyridine (PT) as the acceptor unit and alkoxy thiophene as the donor as well as their corresponding polymers have been successfully synthesized by electropolymerization to understand the effects of different electron donating groups on the electrochemical and optoelectronic properties of the polymers. Electrochemical and spectroelectrochemical characterization demonstrate that methoxythiophene and butoxythiophene were strong electron donors and that the properties of PBOTTP were inferior to those of PMOTTP due to the steric hindrance of the butyl chain. The optical band gaps of PMOTTP and PBOTTP were 0.950 and 1.088 eV, respectively. The corresponding polymer films revealed a saturated green color in the neutral state, a saturated red color in the reduced state and a highly transmissive blue color in the oxidized state, which realized RGB in one polymer. At the same time, the outstanding optical contrasts in the NIR region (approx. 60%), very fast switching times (about 0.5 s) and high environmental stabilities make these polymers paramount choices for the green component of polymer electrochromic display applications. Moreover, the existence of the n-type doping process was proven by both electrochemical and spectral analysis of the films. Efforts to combine new streams of thought for further flowering of this already fertile field are still in progress and the results will be reported in due course.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51473074, 31400044), and the General and Special Program of the Postdoctoral Science Foundation China (2013M530397, 2014T70861).Notes and references
- C. K. Chiang, C. R. Fincher, Y. W. Park, A. J. Heeger, H. Shirakawa, E. J. Louis, S. C. Gau and A. G. MacDiarmid, Phys. Rev. Lett., 1977, 39, 1098–1101 CrossRef CAS.
- C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Adv. Funct. Mater., 2001, 11, 15–26 CrossRef CAS.
- R. J. Mortimer, Chem. Soc. Rev., 1997, 26, 147–156 RSC.
- N. Stutzmann, R. H. Friend and H. Sirringhaus, Science, 2003, 299, 1881–1884 CrossRef CAS PubMed.
- D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537–2574 CrossRef CAS PubMed.
- P. K. H. Ho, D. S. Thomas, R. H. Friend and N. Tessler, Science, 1999, 285, 233–236 CrossRef CAS.
- I. Schwendeman, R. Hickman, G. Sonmez, P. Schottland, K. Zong, D. M. Welsh and J. R. Reynolds, Chem. Mater., 2002, 14, 3118–3122 CrossRef CAS.
- G. Sonmez, H. Meng, Q. Zhang and F. Wudl, Adv. Funct. Mater., 2003, 13, 726–731 CrossRef CAS.
- A. A. Argun, P. H. Aubert, B. C. Thompson, I. Schwendeman, C. L. Gaupp, J. Hwang, N. J. Pinto, D. B. Tanner, A. G. MacDiarmid and J. R. Reynolds, Chem. Mater., 2004, 23, 4401–4412 CrossRef.
- M. M. Verghese, M. K. Ram, H. Vardhan, B. D. Malhotra and S. M. Ashraf, Polymer, 1997, 38, 1625–1629 CrossRef CAS.
- D. N. Buckley and L. D. Burke, J. Chem. Soc., Faraday Trans., 1975, 1, 1447–1459 RSC.
- L. Groenendaal, G. Zotti, P.-H. Aubert, S. M. Waybright and J. R. Reynolds, Adv. Mater., 2003, 15, 855–879 CrossRef CAS.
- S. A. Sapp, G. A. Sotzing and J. R. Reynolds, Chem. Mater., 1998, 10, 2101–2108 CrossRef CAS.
- A. Kumar, D. M. Welsh, M. C. Morvant, K. A. Abboud and J. R. Reynolds, Chem. Mater., 1998, 10, 896–902 CrossRef CAS.
- G. Sonmez, H. B. Sonmez, K. F. Shen, R. W. Jost, Y. Rubin and F. Wudl, Macromolecules, 2005, 38, 669–675 CrossRef CAS.
- P. M. Beaujuge and J. R. Reynolds, Chem. Rev., 2010, 110, 268–320 CrossRef CAS PubMed.
- G. Sonmez, Chem. Commun., 2005, 5251–5259 RSC.
- G. Sonmez, C. K. F. Shen, Y. Rubin and F. Wudl, Angew. Chem., Int. Ed., 2004, 116, 1498–1502 CrossRef PubMed.
- A. Ennisi, F. Simone, G. Barletta, G. Di Marco and L. Lanza, Electrochim. Acta, 1999, 44, 3237–3243 CrossRef.
- J. Mao, J. Yang, J. Teuscher, T. Moehl, C. Yi, R. Humphry-Baker, P. Comte, C. Grätzel, J. Hua, S. M. Zakeeruddin, H. Tian and M. Grätzel, J. Phys. Chem. C, 2014, 118(30), 17090–17099 CAS.
- P. M. Beaujuge, S. Ellinger and J. R. Reynolds, Adv. Mater., 2008, 20, 2772–2776 CrossRef CAS PubMed.
- A. Durmus, G. E. Gunbas, P. Camurlu and L. Toppare, Chem. Commun., 2007, 3246–3248 RSC.
- A. Cihaner and F. Algı, Adv. Funct. Mater., 2008, 18, 3583–3589 CrossRef CAS.
- G. E. Gunbas, A. Durmus and L. Toppare, Adv. Funct. Mater., 2008, 18, 2026–2030 CrossRef CAS.
- G. E. Gunbas, A. Durmus and L. Toppare, Adv. Mater., 2008, 20, 691–695 CrossRef CAS.
- F. Ozyurt, E. G. Gunbas, A. Durmus and L. Toppare, Org. Electron., 2008, 9, 296–302 CrossRef CAS PubMed.
- Y. A. Udum, A. Durmus, G. E. Gunbas and L. Toppare, Org. Electron., 2008, 9, 501–506 CrossRef CAS PubMed.
- D. Baran, G. Oktem, S. Celebi and L. Toppare, Macromol. Chem. Phys., 2011, 212, 799–805 CrossRef CAS.
- N. Blouin, A. Michaud, D. Gendron, S. Wakim, E. Blair, R. Neagu-Plesu, M. Belletête, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732–742 CrossRef CAS PubMed.
- Y. Sun, G. C. Welch, W. L. Leong, C. J. Takacs, G. C. Bazan and A. J. Heeger, Nat. Mater., 2012, 11, 44–48 CrossRef CAS PubMed.
- A. Balan, D. Baran, G. Gunbas, A. Durmus, F. Ozyurt and L. Toppare, Chem. Commun., 2009, 6768–6770 RSC.
- W. Ying, X. Zhang, X. Li, W. Wu, F. Guo, J. Li, H. Ågren and J. Hua, Tetrahedron, 2014, 70, 3901–3908 CrossRef CAS PubMed.
- X. X. Sun, Y. Z. Lou, Q. Q. Chen, L. Li, X. X. Zhuang and Y. C. Li, Adv. Mater. Res., 2011, 335–336, 90–95 CAS.
- Q. Hou, Q. Zhou, Y. Zhang, W. Yang, R. Yang and Y. Cao, Macromolecules, 2004, 37, 6299–6305 CrossRef CAS.
- A. Kraft, A. C. Grimsdale and A. B. Holmes, Angew. Chem., Int. Ed., 1998, 37, 403–428 CrossRef CAS.
- D. M. de Leeuw, M. M. J. Simenon, A. R. Brown and R. E. F. Einerhand, Synth. Met., 1997, 87, 53–59 CrossRef CAS.
- M. İ. Özkut, S. Atak, A. M. Önal and A. Cihaner, J. Mater. Chem., 2011, 21, 5268–5272 RSC.
- D. Baran, G. Oktem, S. Celebi and L. Toppare, Macromol. Chem. Phys., 2011, 212, 799–805 CrossRef CAS.
- H. Akpınar, A. Balan, D. Baran, E. K. Ünver and L. Toppare, Polymer, 2010, 51, 6123–6131 CrossRef PubMed.
- J. J. Aperloo and R. A. J. Janssen, Synth. Met., 1999, 101, 417–420 CrossRef.
- B. Sankaran and J. R. Reynolds, Macromolecules, 1997, 30, 2582–2588 CrossRef CAS.
- A. Cihaner and F. Algı, Electrochim. Acta, 2008, 54, 786–792 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and 13C NMR spectra of the intermediates and target compounds. See DOI: 10.1039/c4ra11628c |
| This journal is © The Royal Society of Chemistry 2014 |