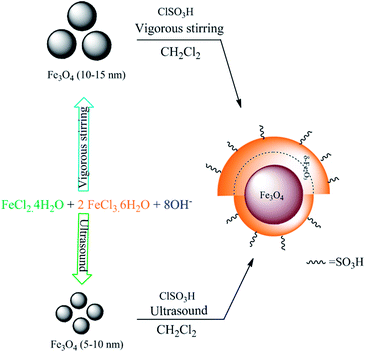
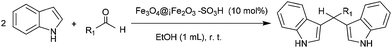Sulfonic acid-functionalized magnetic nanoparticles as a recyclable and eco-friendly catalyst for atom economical Michael addition reactions and bis indolyl methane synthesis†
Hajar Mahmoudia,
Abbas Ali Jafari*a,
Soroosh Saeedia and
Habib Firouzabadi*b
aDepartment of Chemistry, College of Sciences, Yazd University, 89195-741 Yazd, Iran. E-mail: jafari@yazd.ac.ir
bLate Professor Moshfegh Laboratory, Chemistry Department, Shiraz University, Shiraz 71454, Iran. E-mail: firouzabadi@chem.susc.ac.ir
First published on 2nd December 2014
Abstract
A modified sulfonic acid functionalized magnetic nanoparticle composite (Fe3O4@γFe2O3–SO3H) is prepared by the use of ultrasonic irradiation. By this modification, the size of the nano-particles was reduced and the magnetization of the material was highly improved. This improvement drastically affected the efficiency and magnetic separation of the material as a catalyst. This new modified nanomagnetic compound was used as a highly efficient and recyclable catalyst for functionalization of indole derivatives via Michael addition and Friedel–Crafts alkylation reactions in entirely environmentally friendly media at room temperature. This modified magnetic material can be easily applied for large-scale operations producing the desired products in excellent yields in highly pure states.
Introduction
Carbon–carbon bond formation reactions are very important from different aspects, especially in synthetic organic chemistry. In the last decades, research has begun to study the effect of transition metal catalysts and also different types of acidic and basic catalysts with the emphasis on green chemistry.1–9The Michael addition reaction and bisindolyl methane synthesis are important C–C bond forming reactions, which have found wide synthetic applications in material sciences, agrochemicals, and pharmaceuticals.10–12 Several homogeneous acid and base catalysts are used to effect these reactions. In the presence of strong bases or acids, side reactions such as multiple condensations, polymerizations, and rearrangements are common to occur.13–17 Furthermore; many of these procedures have some drawbacks consisting of corrosive and expensive reagents, low yield of products, difficult handling, and long reaction times. To overcome the mentioned difficulties, chemists investigated a wide range of strategies. One of the logical solution for the above mentioned problems is to use heterogeneous catalysts carrying nano-materials (NMs).18–20
Nano scale particles reveal novel properties which are not found in their macroscopic counterparts. These properties contribute to the efficiency and capability of nano-catalysts in the reactions.21–28 However; the major problems in using the nano-catalysts are their isolation and recovery from the reaction mixture. One of the smart ways for the isolation and separation of catalysts can be accomplished by their support on the surface of magnetic nanoparticles (MNPs). By this achievement, the catalyst can be isolated by an external magnet from the reaction mixture by minimum contamination of the products. In addition, by adopting this strategy, easy recycling of the catalyst without using the time consuming filtration step can be achieved.29–32 Undoubtedly, the utilization of MNPs in catalysts separation constitutes the economically and technologically important application that has stimulated vast research efforts in this area.33–36 However, it is noteworthy to state that, the naked metallic nanoparticles with the high surface area to volume ratio are highly chemically active and are easily oxidized in air, resulting generally in loss of magnetism and disperser of MNPs.37 Hence, for this reason, it is important to develop a protection strategy for stabilizing the naked MNPs against degradation during or after the synthesis. This strategy consists of coating the naked magnetic nanoparticles with polymers or an inorganic layer such as gold, silica or carbon.38–43 There is also notable to declare that, the coating protocol may provide useful sites for further functionalization of the material. For instance, the strong acids, known as damaging materials in industry and environment, can be supported on MNPs. Sulfonic acid-functionalized magnetic nanoparticles (SAMN) is known as the recoverable solid strong acid.44–46 The SAMN is of great interest in both academic and industrial communities due to its economically importance and environmentally benign features. Nevertheless, up to now, only a few strategies for the preparation of SAMN have been reported in the literature. Along of our interest for applying SAMN as a catalyst in important synthetic reactions,47 now we report a modification for the preparation of the SAMN by using ultrasound irradiation instead of vigorous mechanical steering.47 By this modification a promising advancement in magnetic separation of the material, smaller size of the nano-particles and as a result higher catalytic activity has been observed. This modified SAMN has been used as an effective catalyst for the high yielding eco-friendly processes for the preparation of Michael adducts and also for the preparation of 3-substituted indolyl derivatives via Friedel–Crafts alkylation reaction of indole.
Results and discussion
The preparation of the modified SAMN material was accomplished by the assistance of ultrasound irradiation. The effect of using ultrasound upon the size of the particles from 10–15 nm (ref. 47) to 5–10 nm has been a noticeable phenomenon. This particle size reduction affects the magnetic separation of the material much easier and faster in comparison with our previously reported SAMN47 (Scheme 1).The new SAMN was characterized by XRF, TEM, and VSM and the amounts of sulfonic acid functional group on magnetic nanoparticles with maghemite coating supports was determined by the neutralization titration method to be in the range of 4–4.5 mmol g−1.
X-ray fluorescence analysis (Table 1) confirms the presence of sulfonic acid on the catalyst surface. The XRF analysis proves that the use of ultrasonic irradiation, instead of vigorous stirring in sulfonation process of MNPs, reduces the conversion of Fe3O4 to γFe2O3. The amount of γFe2O3 on the outer layer of Fe3O4 is less than that of our previously reported procedure47 (Fig. 1).
| Compound | Concentrationb (%W/W) | Concentrationc (%W/W) |
|---|---|---|
| a Loss on ignition (1000 °C, 2 h).b Ultrasonic irradiation.c Vigorous stirring. | ||
| Fe2O3 | 23.50 | 42.65 |
| Fe3O4 | 20.05 | 0 |
| SO3 | 19.06 | 20.26 |
| Cl | 2.01 | 1.66 |
| V2O5 | 0.060 | 0.073 |
| Al2O3 | 0.031 | 0.041 |
| MnO | 0.027 | 0.034 |
| CaO | 0.019 | 0.029 |
| CuO | 0.010 | 0.020 |
| ZnO | 0.009 | 0.009 |
| LOIa | 35.28 | 35.48 |
| Total | 100.05 | 100.26 |
 | ||
| Fig. 1 XRF analysis of SAMN (a) by vigorous steering47 (b) under ultrasonic irradiation. | ||
The results of transmission electron microscopy (TEM) analysis of the SAMN demonstrate uniform-sized particles with spherical morphology with an average size range of 5–10 nm (Fig. 2). This also shows the effect of ultrasonic irradiation upon the size of the particles with respect to vigorous stirring (10–15 nm).47
The magnetization curves of the Fe3O4 and modified SAMN were further recorded at room temperature (Fig. 3). The saturation magnetization value of the modified SAMN is 90 emu g−1 (Fig. 3b). The number is smaller than that of uncoated magnetic nanoparticles which is 93 emu g−1 (Fig. 3a) and becomes nearly 1.63 times greater than that of our previous report for the preparation of Fe3O4@γFe2O3–SO3H47 which is 55 emu g−1 (Fig. 3c) and nearly 3.2 times greater than γFe2O3–SO3H46 that is 28 emu g−1 (Fig. 3d).
 | ||
| Fig. 3 Magnetization loops of (a) Fe3O4, (b) modified SAMN (c) previously reported SAMN47 (d) γFe2O3–SO3H.46 All the data are recorded at room temperature. | ||
The magnetization curve of the modified SAMN assures that the use of ultrasonic irradiation produces an agile SAMN which accumulates in a blink by an external magnetic field from water (Fig. 4).
 | ||
| Fig. 4 (a) Dispersed modified SAMN in water (b) fast separation of the modified SAMN with a magnet bar from water. | ||
This modified material has been successfully applied as a catalyst for Michael addition and Friedel–Crafts reactions. In order to optimize the reaction conditions with respect to temperature, time, and the molar ratio of the substrates, the reaction of indole with methyl vinyl ketone as a model reaction was studied. The reaction was proceeded sluggishly and after a prolonged reaction time (5 h), the corresponding Michael adduct was produced in only 20% yield in the absence of the catalyst. Then, similar reaction was performed in the presence of different mol% of the modified SAMN. We observed a drastic rate enhancement under solvent free conditions and also in different solvents. Promising results were obtained at room temperature when solvent free condition or solvents such as EtOH, CH3CN, n-hexane, CH2Cl2 and acetone were used (Table 2).
| Entry | Cat. (mol%) | Solvent | Temp. (°C) | Time (min) | Conversion (%) |
|---|---|---|---|---|---|
| 1 | — | — | 25 | 300 | 20 |
| 2 | Fe3O4 (0.07 g) | — | 25 | 300 | 100 |
| 3 | Fe3O4@γFe2O3–SO3H (5) | — | 25 | 30 | 100 |
| 4 | Fe3O4@γFe2O3–SO3H (7) | — | 25 | 20 | 100 |
| 5 | Fe3O4@γFe2O3–SO3H (10) | — | 25 | 10 | 100 |
| 6 | Fe3O4@γFe2O3–SO3H (15) | — | 25 | 5 | 100 |
| 7 | Fe3O4@γFe2O3–SO3H (10) | EtOH | 25 | 180 | 100 |
| 8 | Fe3O4@γFe2O3–SO3H (10) | H2O | 25 | 300 | 30 |
| 9 | Fe3O4@γFe2O3–SO3H (10) | H2O | 100 | 300 | 70 |
| 10 | Fe3O4@γFe2O3–SO3H (10) | PEG | 25 | 300 | 20 |
| 11 | Fe3O4@γFe2O3–SO3H (10) | n-Hexane | 25 | 150 | 100 |
| 12 | Fe3O4@γFe2O3–SO3H (10) | CH3CN | 25 | 120 | 100 |
| 13 | Fe3O4@γFe2O3–SO3H (10) | CH2Cl2 | 25 | 120 | 100 |
| 14 | Fe3O4@γFe2O3–SO3H (10) | Acetone | 25 | 240 | 100 |
In order to show the general applicability of the method, the reaction of various indoles with α,β-unsaturated carbonyl compounds under solvent free conditions was studied. The results of this study are tabulated in Table 3.
| Entry | Product | Time (min) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: indole (1 mmol), α,β-unsaturated carbonyl compound (1.1 mmol) and modified SAMN (0.1 mmol, 0.03 g) were mixed and mechanically stirred at room temperature. | |||
| 1 |  |
10 | 95 |
| 2 |  |
60 | 90 |
| 3 |  |
180 | 93 |
| 4 |  |
240 | 90 |
| 5 |  |
10 | 97 |
| 6 |  |
5 | 95 |
| 7 |  |
120 | 97 |
| 8 |  |
60 | 98 |
In continuation of our studies, Friedel–Crafts alkylation reaction of indole with an aldehyde was also investigated in the presence of the modified catalyst. At first, the reaction of benzaldehyde with indole was studied as a model reaction to optimize the reaction conditions. In the absence of the catalyst, 3,3′-(phenylmethylene)bis(1H-indole) was produced in only 30% yield after a long reaction time (24 h). In the presence of the modified catalyst, the reaction was progressed well at room temperature and the desired bisindolyl methane was produced rapidly (15 min) in a quantitative yield in solvents such as EtOH, CH3CN, CH2Cl2 and petroleum ether at different reaction times (Table 4).
| Entry | Cat. (mol%) | Solvent | Tem. (°C) | Time (h) | Conversion (%) |
|---|---|---|---|---|---|
| 1 | — | EtOH | 25 | 24 | 30 |
| 2 | Fe3O4 | EtOH | 25 | 5 | 100 |
| 3 | Fe3O4@γFe2O3–SO3H (5) | EtOH | 25 | 1 | 100 |
| 4 | Fe3O4@γFe2O3–SO3H (7) | EtOH | 25 | 0.5 | 100 |
| 5 | Fe3O4@γFe2O3–SO3H (10) | EtOH | 25 | 0.33 | 100 |
| 6 | Fe3O4@γFe2O3–SO3H (15) | EtOH | 25 | 0.25 | 100 |
| 7 | Fe3O4@γFe2O3–SO3H (10) | H2O | 25 | 24 | 50 |
| 8 | Fe3O4@γFe2O3–SO3H (10) | H2O | 100 | 5 | 60 |
| 9 | Fe3O4@γFe2O3–SO3H (10) | PEG | 25 | 24 | 30 |
| 10 | Fe3O4@γFe2O3–SO3H (10) | CH3CN | 25 | 2.5 | 100 |
| 11 | Fe3O4@γFe2O3–SO3H (10) | CH2Cl2 | 25 | 6 | 100 |
| 12 | Fe3O4@γFe2O3–SO3H (10) | Petroleum ether | 25 | 8 | 100 |
However, in order to show the advancement of the catalytic activity of the ultrasonically assisted preparation of SAMN with our previously reported SAMN,47 the reaction of benzaldehyde with indole was studied (Scheme 2). As it is evident from the results, the catalytic activity of the modified SAMN is considerably 18 times greater than the non-modified one.
To demonstrate the general widespread application of the method, the addition of structurally different indoles to aldehydes in EtOH at room temperature was studied. The results of this study are listed in Table 5.
| Entry | Product | Time (min) | Yield (%) |
|---|---|---|---|
| a Reaction conditions: indole (2 mmol), aldehyde (1 mmol) and modified SAMN (0.1 mmol, 0.03 g) and ethanol (1 mL) were mechanically stirred at room temperature. | |||
| 1 |  |
20 | 95 |
| 2 | 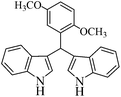 |
30 | 91 |
| 3 |  |
10 | 95 |
| 4 |  |
20 | 94 |
| 5 |  |
20 | 90 |
| 6 |  |
15 | 93 |
| 7 |  |
5 | 93 |
| 8 |  |
60 | 98 |
The high purity of the products with respect to the iron contamination has been approved by manganometry titration analysis.
Considering the importance of using catalysts for the large scale operation and their further applications in industry, we have studied the use of the catalyst in large scale Michael addition and Friedel–Crafts alkylation reactions with success under solvent free condition and in EtOH respectively (Scheme 3 and 4).
 | ||
| Scheme 3 Michael addition of indole to methyl vinyl ketone in large scale catalyzed by modified SAMN catalyst. | ||
Efficient recycling of the catalyst is important from environmental and economic point of views. Therefore, recycling of this catalyst was studied for the reaction of indole with methyl vinyl ketone (Fig. 5). After completion of each run, the catalyst was separated by an external magnetic bar and then was reused for another batch of the reaction. This process has been repeated for 5 consecutive runs without observable decrease in catalytic activity of the catalyst (Fig. 5). This observation shows that the morphology and also the size of the particles of the catalyst should not be changed during different runs. In order to approve this statement, TEM image of the catalyst after the first run of recycling (Fig. 6) was compared with the TEM image of the catalyst before reaction (Fig. 2) It shows that the size (10–15 nm) and the morphology (spherical) of the catalyst has been preserved during the reaction.
 | ||
| Fig. 5 Recycling of the modified catalyst for 5 consecutive runs for Michael addition reaction of indole with methyl vinyl ketone. | ||
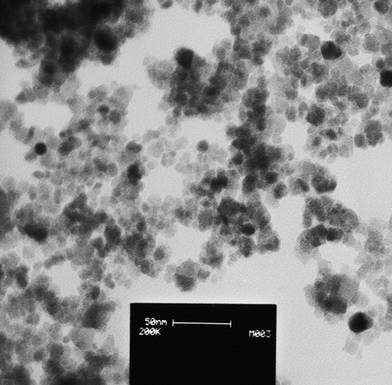 | ||
| Fig. 6 TEM image of the catalyst after the 1st run of recycling shows that the size (10–15 nm) and the morphology (spherical) of the catalyst nano particles are preserved. | ||
Experimental section
General experimental methods
All commercially available chemicals were obtained from Merck and Fluka Chemical Companies, and used without further purification unless otherwise stated; solvents were distilled before their use. The products were characterized using FTIR, 1HNMR (500 MHz) and 13CNMR (125 MHz) spectroscopy. All the reactions are monitored by thin layer chromatography (TLC) coated with silica gel illuminated with UV light and iodine as the reagent. All yields refer to the isolated products.Preparation of the modified Fe3O4 nanoparticles (NPs)
The Fe3O4 NPs were prepared by using the published method48 with modification. FeCl3·6H2O (20 mmol) and FeCl2·4H2O (10 mmol) were added to deionized water (4 mL) under nitrogen atmosphere with ultrasound irradiation for 15 min at 60 °C. Then, ammonium hydroxide (15 mL, 28 wt%) was added rapidly to the resulting solution. The solution was immediately turned black. The reaction was kept at 60 °C for 20 min under ultrasound irradiation. The black precipitates were collected with an external magnet and washed with distilled water. The resulting Fe3O4 NPs were dried for 12 h at room temperature under vacuum and characterized by XRD, FTIR.Preparation of the SAMN
A suction flask was equipped with a constant-pressure dropping funnel and a gas inlet for leading the generated HCl into water. To this flask, which contains nano-particles of Fe3O4 (1.00 g) as described in the preceding paragraph in dichloromethane (20 mL), cholorosulfonic acid (1.0 mL) was added drop wise at room temperature for 15 min under ultrasound irradiation. The resulting mixture was sonicated until HCl gas evolution was stopped. The resulting modified SAMN material was separated by an external magnet and washed with dichloromethane (3 × 5 mL) and finally dried in an oven at 40 °C. A brown solid of the modified Fe3O4@γFe2O3–SO3H was obtained that was characterized by XRF, TEM, FTIR.General procedure for the one-pot reaction of indole with α,β-unsaturated carbonyl compounds
Modified SAMN (10 mol%, 0.03 g) was added to a stirring mixture of indole (1 mmol, 0.117 g) and α,β-unsaturated carbonyl compounds (1.1 mmol) at room temperature. The progress of the reaction was monitored by TLC (hexane–ethylacetate eluent 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After completion of the reaction, ethanol (10 mL) was added to the reaction mixture. The catalyst was separated by a permanent magnet from the reaction mixture. The desired Michael adducts were obtained after concentration of the reaction mixture under diminished pressure as a highly pure crystalline or oily compounds in good to excellent yields (Table 3).
1). After completion of the reaction, ethanol (10 mL) was added to the reaction mixture. The catalyst was separated by a permanent magnet from the reaction mixture. The desired Michael adducts were obtained after concentration of the reaction mixture under diminished pressure as a highly pure crystalline or oily compounds in good to excellent yields (Table 3).
General procedure for bisindolyl methane synthesis
A mixture of indole (2 mmol, 0.234), aldehyde (1 mmol) and modified SAMN (10 mol%, 0.03 g) in ethanol (1 mL) was mechanically stirred at room temperature. After completion of the reaction (TLC, hexane–ethylacetate, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the resulting mixture was diluted with ethanol (15 mL) and the solid catalyst was separated by a permanent magnet from the reaction mixture. Upon evaporation of ethanol under vacuum from the resulting solution, the desired pure crystalline product was obtained in high to excellent yields (Table 5).
1), the resulting mixture was diluted with ethanol (15 mL) and the solid catalyst was separated by a permanent magnet from the reaction mixture. Upon evaporation of ethanol under vacuum from the resulting solution, the desired pure crystalline product was obtained in high to excellent yields (Table 5).
Conclusions
In conclusion, the preparation of the modified SAMN material was accomplished by the assistance of ultrasound irradiation. The effect of using ultrasound upon the size of the particles from 10–15 nm (our previous work)47 to 5–10 nm has been a noticeable phenomenon. This particle size reduction affects the magnetic separation of the material much easier and faster in comparison with the previously reported SAMN.47 The agile magnetic hydrogen sulfate nanoparticles produced under the influence of ultrasound irradiation accumulate in a blink with an external magnetic field from the reaction mixture. This magnetic separation is facile and eliminates time consuming filtration process. In the presence of this modified catalyst under solvent free condition, Michael addition of indols with α,β-unsaturated carbonyl compounds proceeded well and produced the desired highly pure Michael addition adducts in excellent yields. The modified SAMN is also an effective catalyst for Friedel–Crafts alkylation reaction. For this purpose, the reaction of indoles with aldehydes resulted bisindolyl methane in excellent yields in ethanol as a green solvent. The protocol is applicable for large scale operation without difficulties. The catalyst is recyclable with preservation of its catalytic activity after several reaction runs. The modified catalyst activity, as observed in a model reaction, is much higher, 18 times, than our previously reported SAMN.47Acknowledgements
The authors would like to thank the Research Council of Yazd University for financial support of this work. H.F. is also thankful for a grant (BN048) from the National Elite Foundation of Iran.References
- D. Basavaiah, P. Dharma Rao and R. Suguna Hyma, Tetrahedron, 1996, 52, 8001–8062 CrossRef CAS.
- K. Sakthivel, W. Notz, T. Bui and C. F. Barbas, J. Am. Chem. Soc., 2001, 123, 5260–5267 CrossRef CAS PubMed.
- H. Mayr, B. Kempf and A. R. Ofial, Acc. Chem. Res., 2002, 36, 66–77 CrossRef PubMed.
- H. Firouzabadi, N. Iranpoor and F. Nowrouzi, Chem. Commun., 2005, 789–791 RSC.
- H. Firouzabadi, N. Iranpoor, M. Jafarpour and A. Ghaderi, J. Mol. Catal. A: Chem., 2006, 252, 150–155 CrossRef CAS PubMed.
- A. A. Jafari, F. Moradgholi and F. Tamaddon, J. Iran. Chem. Soc., 2009, 6, 588–593 CrossRef CAS.
- B. M. Monks and S. P. Cook, Angew. Chem., 2013, 125, 14464–14468 CrossRef.
- J. K. Chavda, P. A. Procopiou, P. N. Horton, S. J. Coles and M. J. Porter, Eur. J. Org. Chem., 2014, 2014, 129–139 CrossRef CAS PubMed.
- S. Doherty, J. G. Knight, J. R. Ellison, P. Goodrich, L. Hall, C. Hardacre, M. J. Muldoon, S. Park, A. Ribeiro, C. A. N. de Castro, M. J. Lourenco and P. Davey, Green Chem., 2014, 16, 1470–1479 RSC.
- D. A. Oare and C. H. Heathcock, J. Org. Chem., 1990, 55, 157–172 CrossRef CAS.
- B. List, P. Pojarliev and H. J. Martin, Org. Lett., 2001, 3, 2423–2425 CrossRef CAS PubMed.
- A. Alexakis and O. Andrey, Org. Lett., 2002, 4, 3611–3614 CrossRef CAS PubMed.
- J. S. Yadav, B. V. Subba Reddy, C. V. S. R. Murthy, G. Mahesh Kumar and C. Madan, Synthesis, 2001, 2001, 0783–0787 CrossRef PubMed.
- C. Ramesh, J. Banerjee, R. Pal and B. Das, Adv. Synth. Catal., 2003, 345, 557–559 CrossRef CAS.
- J. Li, M. Zhou, B. Li and G. Zhang, Synth. Commun., 2004, 34, 275–280 CrossRef CAS PubMed.
- S. Palaniappan and A. John, J. Mol. Catal. A: Chem., 2005, 242, 168–172 CrossRef CAS PubMed.
- N. Azizi, L. Torkian and M. R. Saidi, J. Mol. Catal. A: Chem., 2007, 275, 109–112 CrossRef CAS PubMed.
- K. R. Kloetstra and H. van Bekkum, J. Chem. Soc., Chem. Commun., 1995, 1005–1006 RSC.
- Y. V. S. Rao, D. E. De Vos and P. A. Jacobs, Angew. Chem., Int. Ed. Engl., 1997, 36, 2661–2663 CrossRef CAS.
- M. V. Abolfazl Olyaei, R. Razeghi and H. B. Bahareh Shams, J. Serb. Chem. Soc., 2013, 78, 463–468 CrossRef.
- A. T. Bell, Science, 2003, 299, 1688–1691 CrossRef CAS PubMed.
- J. Grunes, J. Zhu and G. A. Somorjai, Chem. Commun., 2003, 2257–2260 RSC.
- R. Schlögl and S. B. Abd Hamid, Angew. Chem., Int. Ed., 2004, 43, 1628–1637 CrossRef PubMed.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS PubMed.
- J. P. Wilcoxon and B. L. Abrams, Chem. Soc. Rev., 2006, 35, 1162–1194 RSC.
- G. A. Somorjai, H. Frei and J. Y. Park, J. Am. Chem. Soc., 2009, 131, 16589–16605 CrossRef CAS PubMed.
- V. Polshettiwar, B. Baruwati and R. S. Varma, ACS Nano, 2009, 3, 728–736 CrossRef CAS PubMed.
- S. Wittmann, A. Schätz, R. N. Grass, W. J. Stark and O. Reiser, Angew. Chem., Int. Ed., 2010, 49, 1867–1870 CrossRef CAS PubMed.
- A.-H. Lu, E. L. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743–754 RSC.
- S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef CAS PubMed.
- H. Firouzabadi, N. Iranpoor, M. Gholinejad, S. Akbari and N. Jeddi, RSC Adv., 2014, 4, 17060–17070 RSC.
- J. Deng, L.-P. Mo, F.-Y. Zhao, L.-L. Hou, L. Yang and Z.-H. Zhang, Green Chem., 2011, 13, 2576–2584 RSC.
- H. Firouzabadi, N. Iranpoor, M. Gholinejad and J. Hoseini, Adv. Synth. Catal., 2011, 353, 125–132 CrossRef CAS.
- Y.-H. Liu, J. Deng, J.-W. Gao and Z.-H. Zhang, Adv. Synth. Catal., 2012, 354, 441–447 CrossRef CAS.
- R. B. Nasir Baig and R. S. Varma, Green Chem., 2013, 15, 398–417 RSC.
- B. Vincent, J. Edwards, S. Emmett and A. Jones, Colloids Surf., 1986, 18, 261–281 CrossRef CAS.
- H. K. Xu, C. M. Sorensen, K. J. Klabunde and G. C. Hadjipanayis, J. Mater. Res., 1992, 7, 712–716 CrossRef CAS.
- P. Mulvaney, L. M. Liz-Marzan, M. Giersig and T. Ung, J. Mater. Chem., 2000, 10, 1259–1270 RSC.
- S. Santra, R. Tapec, N. Theodoropoulou, J. Dobson, A. Hebard and W. Tan, Langmuir, 2001, 17, 2900–2906 CrossRef CAS.
- J. Lin, W. Zhou, A. Kumbhar, J. Wiemann, J. Fang, E. E. Carpenter and C. J. O'Connor, J. Solid State Chem., 2001, 159, 26–31 CrossRef CAS.
- A. M. Morawski, P. M. Winter, K. C. Crowder, S. D. Caruthers, R. W. Fuhrhop, M. J. Scott, J. D. Robertson, D. R. Abendschein, G. M. Lanza and S. A. Wickline, Magn. Reson. Med., 2004, 51, 480–486 CrossRef CAS PubMed.
- C. Zhang, B. Wängler, B. Morgenstern, H. Zentgraf, M. Eisenhut, H. Untenecker, R. Krüger, R. Huss, C. Seliger, W. Semmler and F. Kiessling, Langmuir, 2006, 23, 1427–1434 CrossRef PubMed.
- C. S. Gill, B. A. Price and C. W. Jones, J. Catal., 2007, 251, 145–152 CrossRef CAS PubMed.
- L. Ma'mani, M. Sheykhan, A. Heydari, M. Faraji and Y. Yamini, Appl. Catal., A, 2010, 377, 64–69 CrossRef PubMed.
- N. Koukabi, E. Kolvari, M. A. Zolfigol, A. Khazaei, B. S. Shaghasemi and B. Fasahati, Adv. Synth. Catal., 2012, 354, 2001–2008 CrossRef CAS.
- H. Mahmoudi and A. A. Jafari, ChemCatChem, 2013, 5, 3743–3749 CrossRef CAS.
- J.-P. Jolivet, C. Chaneac and E. Tronc, Chem. Commun., 2004, 481–483 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra11605d |
| This journal is © The Royal Society of Chemistry 2015 |