Design of multifunctional FePt/GO nanocomposites for targeting, dual-modal imaging diagnostic and in situ therapeutic potential theranostic platform†
Xiuwen Zheng*ab,
Weihong Chenab,
Ping Cuia,
Zhiming Wanga and
Wei Zhangab
aSchool of Chemistry & Chemical Engineering, Linyi University, China
bShandong Provincial Key Laboratory of Detection Technology for Tumor Makers, Linyi University, China. E-mail: xwzheng1976@gmail.com
First published on 20th October 2014
Abstract
In this work, highly monodispersed chemically disordered face centered cubic (fcc) FePt nanoparticles (NPs) were assembled on graphene oxide (GO) surface to form FePt/GO nanocomposites via a simple polyol protocol. Conjugated with the biocompatible 6-arm polyethylene glycol-amine polymer, folic acid and fluorescein isothiocyanate (FITC), the as-prepared nanocomposites exhibit high stability in physiological solutions, targeted delivery to folate receptor-positive cancer cells and dual-modal visualization of cellular uptake by fluorescence (FITC) and magnetic resonance imaging. As pH-sensitive agents, the fccFePt NPs display high cytotoxicity because of the generation of highly reactive oxygen species within cancer cells. Due to their targeting, diagnostic and therapeutic functions, the FePt/GO nanocomposites are therefore promising for potential theranostic applications in cancer treatment.
1. Introduction
During the past few decades, one of the most important emerging multidisciplinary areas in nanotechnology is cancer nanotechnology, which aims to develop effective nanotheranostic agents for the longstanding problems in cancer early diagnosis and therapy.1–5 In contrast to separate nanomaterials for therapy and diagnosis, “nanotheranostics” have the potential to overcome undesirable difficulties in biodistribution and selectivity that currently exist between distinct imaging and therapeutic agents.3 The ultimate goal is to obtain desired theranostic agents with the ability of targeting, multimodality imaging diagnosis of diseased tissue and simultaneously treatment with as few side effects as possible.2,6 Therefore, great progress has been made in the research of theranostic agents. For example, Ke et al. designed a multifunctional theranostic agent composed of gold-nanoshelled microcapsules (GNS-MCs), and ultrasound-responsive polymeric microcapsules for systemic contrast-enhanced ultrasound imaging diagnosis have been successfully constructed, which hold great potential for ultrasound-guided photothermal tumor therapy.7 Gao et al. conjugated Au and γ-Fe2O3 NPs with RGD and FITC-DEVD to achieve preferential binding to integrin ανβ3-rich human liver cancer cells (HepG2), enabling the catalytic formation of hydroxyl radicals (˙OH) and real-time monitoring of ˙OH-induced caspase-3-dependent apoptosis in these cancer cells.8 Using a layer-by-layer self-assembly approach, Chen et al. prepared a novel class of multifunctional nanocomposites with superparamagnetic iron oxide nanoparticles, upconversion nanoparticles (UCNPs), gold shell for in vitro targeted upconversion luminescence, magnetic resonance (MR), and dark-field scattering multimodal imaging of cells.9 The near infrared (NIR) optical absorption offered by the gold shell also enables molecular and magnetic dual-targeted photothermal destruction of cancer cells. And also, hexagonal phase NaYF4:Yb,Er/NaGdF4 core–shell UCNPs conjugated with Ce6, a photodynamic therapy (PDT) drug, have been successfully prepared and used not only as dual-modal imaging probes for accurate diagnosis but also as PDT agents for efficient therapy.10 All of the aforementioned works present a unique strategy for multimodal imaging guided, magnetically targeted physical cancer therapy and highlight the promise of using multifunctional nanostructures for novel cancer theranostics.Recently, monodispersed FePt NPs have attracted considerable attention due to their fascinating potential applications in biomedical sciences as CT/MRI molecular imaging contrast agents,11,12 in drug delivery,13,14 on biosensing,15 etc. Also, some exciting results have been reported of FePt NPs having potential therapeutic functions for cancer therapy as chemotherapeutic agents16 or hyperthermia agents17,18 due to the release of Fe or the photothermal and magnetic-thermal effects activated by a NIR femtosecond laser or external magnetic fields, respectively. Thus, FePt-based nanocomposites present an opportunity in realizing CT, MRI, magnetic-thermal, and photothermal treatments all within a single agent, and will open up a new strategy to develop novel anticancer nanomedicine agents with both diagnostic and therapeutic functions.
As is well known, graphene oxide (GO) can be functionalized with biocompatible polymers such as polyethylene glycol (PEG), to acquire improved stability in physiological environments. Functionalized nanoscale GO bioconjugates have been widely explored as drug and gene carriers.19,20,31 Here, combining the merits of FePt (pH-responsive FePt “chemotherapy”) and GO (functionalized with biocompatible polymers), we aim to design a new class of theranostic agent with targeting, imaging and therapeutic functions. FePt/GO nanocomposites were synthesized by a simple polyol route and then functionalized with the biocompatible PEG polymer. Folic acid (FA) and fluorescein isothiocyanate (FITC) were conjugated onto the as-prepared FePt/GO–PEG nanocomposites for the effective targeting of folate receptor-rich tumor cells and the dual-modal visualization of cellular uptake by fluorescence (FITC) and magnetic resonance imaging. After cell uptake, the fccFePt nanoparticles (NPs) display high cytotoxicity due to the generation of reactive oxygen species (ROS) within cells. Due to their targeting, diagnostic and therapeutic functions, the carbon-based FePt nanocomposites are therefore promising for future nanotheranostic applications in cancer treatment.
2. Experimental section
2.1 Materials
General: analytical reagents, such as FeCl2·4H2O, H2PtCl6·6H2O, NaOH, N-hydroxysuccinimide (NHS), FA, FITC, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC), and tetraethylene glycol, were all purchased from J&K Chemical Ltd. Spin column was purchased from Beyotime Institute of Biotechnology. Six-arm polyethylene glycol-amine (10 kDa) was purchased from SunBio, Inc. Single-layer GO was made by a modified Hummers method starting from graphite.212.2 Synthesis of FePt/GO nanocomposites
The FePt/GO nanocomposites were synthesized as in our previously reported route with a slight modification.22 In a typical synthesis, GO (20 mg) was completely dispersed into tetraethylene glycol with the assistance of sonication. After that, FeCl2·4H2O (0.15 g, 0.75 mmol) and H2PtCl6·6H2O dispersed in tetraethylene glycol solution (10 mL, 3.86 × 10−5 mol mL−1) and NaOH (0.15 g, 3.175 mmol) were added into the solution under sonication. The mixed solutions were heated up to ca. 260 °C and kept at this temperature for 1 h under a nitrogen atmosphere. The products were collected by centrifugation, and rinsed several times with deionized (DI) water. The final products were dispersed into PBS (pH = 7, ca. 5 mg mL−1) solution.2.3 Surface modification of FePt/GO NPs with PEG-amine
Briefly, 100 mg of 6-arm PEG amine, 200 mg EDC and 100 mg NHS were added into FePt/GO solutions (50 mL, 5 mg mL−1) and ultrasonicated for 10 min. The reaction solution was stirred overnight at room temperature. The resulting product was purified by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rev min−1 for 20 min and washed three times with DI water to remove unreacted PEG and other reagents.
000 rev min−1 for 20 min and washed three times with DI water to remove unreacted PEG and other reagents.
2.4 Conjugation of FA and FITC onto FePt/GO–PEG NPs
FA (30 mg) and FITC (20 mg, dissolved in DMSO, 2 mg mL−1) were added into FePt/GO–PEG PBS solutions (10 mL, 2 mg mL−1) with the assistance of sonication for 30 min. After that, 200 mg of EDC and 100 mg of NHS were added to the solution. The solution was stirred overnight at room temperature. The resulting products were purified by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rev min−1 for 20 min and washed several times with DI water to remove unreacted FA, FITC, and other reagents. The obtained products were denoted as FePt/GO–PEG–FA–FITC. The final products were redispersed into DI water for further application. The final concentrations (Fe content) are 661.7 μg mL−1 by inductively coupled plasma (ICP) measurement.
000 rev min−1 for 20 min and washed several times with DI water to remove unreacted FA, FITC, and other reagents. The obtained products were denoted as FePt/GO–PEG–FA–FITC. The final products were redispersed into DI water for further application. The final concentrations (Fe content) are 661.7 μg mL−1 by inductively coupled plasma (ICP) measurement.
2.5 Cell line and cell culture
MCF-7 and A549 cell lines were kindly provided by Professor Haiyan Liu, Soochow University. Cells were cultured in DMEM with 10% fetal bovine serum and incubated in a humidified atmosphere at 37 °C with 5% CO2.2.6 Confocal imaging of cells
The as-prepared FePt/GO–PEG–FA–FITC NPs (20 μg mL−1 for Fe concentration) were incubated with MCF-7 and A549 cells in 24-well plates for 12 h at 37 °C. Finally, fluorescence microscopy images were captured by a laser confocal microscope.2.7 Characterization of ROS formation in vitro
The MCF-7 cells were seeded in 6-well microplates and incubated for 20 min with DCFH-DA, and then washed three times with fresh DMEM supplemented with 10% fetal bovine serum and treated with the FePt/GO–PEG–FA–FITC composites at a final Fe concentration of each of 20 μg mL−1, 60 μg mL−1, and 100 μg mL−1. After 6 h of cells-NPs incubation, the MCF-7 cells were characterized by fluorescence spectrophotometry (excited at 488 nm). Data were expressed as mean ± SD. Differences in ROS generation between cells treated with NPs and controls were considered statistically significant performing a Student's t-test with a p-value < 0.05.2.8 Effect of the NPs on chromosomal DNA fragmentation
The effect of the NPs on chromosomal DNA fragmentation was investigated with a DNA Ladder Extraction Kit with Spin Column. First, the MCF-7 cells were seeded in cell culture dishes and incubated for 24 h with the FePt/GO–PEG–FA–FITC composites at a final Fe concentration of 20 μg mL−1, 60 μg mL−1, and 100 μg mL−1, then washed three times with fresh DMEM supplemented without 10% fetal bovine serum. The MCF-7 cells were collected into 1.5 mL centrifuge tube for DNA ladder extraction. DNA fragments obtained by this method were loaded onto a 1.5% (w/v) agarose horizontal gel. Agarose gel electrophoresis was performed in TBE buffer (pH 8.0) at 15 V cm−1. The gel was visualized by staining with ethidium bromide and photographed. DNA standard mark DL2000 (Takara Biotechnology Co. Ltd) was used as a DNA size marker.2.9 In vitro cytotoxicity assay
WST assay was performed to evaluate the cytotoxicity of FePt/GO–PEG–FA–FITC composites. The MCF-7 cells were seeded in 96-well plates at a density of 1 × 104 cells in 100 μL culture medium and maintained for 24 h. Then, cells were incubated for 24 h with the FePt/GO–PEG–FA–FITC composites at different Fe concentration of 1 μg mL−1, 5 μg mL−1, 15 μg mL−1, 50 μg mL−1, 75 μg mL−1, and 100 μg mL−1, then washed with PBS buffer three times and added fresh DMEM supplemented with 10% fetal bovine serum. The relative cellular viability was examined by the WST assay. The data were presented as mean ± SD.2.10 Prussian blue staining of cells
The MCF-7 cells were seeded in 96-well plates then incubated with 20 μg mL−1 FePt/GO–PEG–FA–FITC for 24 h. The cells were then fixed with 4% paraformaldehyde for 15 min and incubated with Perls reagent (4% potassium ferrocyanide and 12% HCl, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) for 30 min at room temperature under agitation. The cells were then rinsed well with PBS.
50 v/v) for 30 min at room temperature under agitation. The cells were then rinsed well with PBS.
2.11 Ultrathin sections of cells
The MCF-7 cells were seeded in 6-well plates. The cells were then treated with 20 μg mL−1 FePt/GO–PEG–FA–FITC for 10 h. The cells were collected via trypsin treatment and pelleted at 3500 rpm for 15 min. The pellets were fixed using 4% paraformaldehyde and routinely processed for ultrathin sectioning. The ultrathin sections were prepared using an ultramicrotome, routinely stained with uranyl acetate and lead citrate, and examined using transmission electron microscopy (TEM).2.12 In vitro MR imaging
The minimum detectable iron concentration was obtained by incubation of separate batches of cells (5 × 105 cells per mL) at different iron concentration of 5, 10, 15, and 20 μg mL−1 for 24 h. Imaging parameters of T2-weighted images (T2WI) were investigated by multi slice multi echo (MSME) experiments with an 11.7 T Bruker micro 2.5 micro-MRI system with repetition time (TR) = 5000 ms, echo time (TE) = 80 ms, imaging matrix = 128 × 128, slice thickness = 1 mm, and field of vision (FOV) = 2.5 cm × 2.5 cm.2.13 Instrumentation
The morphology and composition of NPs were characterized by a TEM instrument (JEM-2100, JEOL, Japan) equipped with an energy dispersive spectrometer. Magnetic measurement was carried out at room temperature with a magnetic property measurement system (MPMS) (SQUID VSM, Quantum Design, USA). Ultraviolet and visible spectra were obtained with a UV-Vis spectrometer (Cary 60, Agilent, USA). The iron concentration was measured using an inductively coupled plasma optical emission spectrometer (ICP-OES, VISTA-MPX, Varian, USA). WST assay was performed with a microplate reader (Eon, Biotek, USA). Cell lines were cultured in a water-jacketed CO2 incubator (3111, Thermo, USA). MRI was carried out with an 11.7 T micro 2.5 micro-MRI system (Avance 500WB, Bruker, Germany) with a conventional spin echo acquisition. Relaxivity (γ2) with units of mM−1 s −1 was calculated through the curve-fitting of the reciprocal of the relaxation time versus the iron concentration (mM Fe). Cell slices were obtained by a cell slicing machine (EMUC6, Leica, Germany). Fluorescence microscopy images were captured by a laser confocal microscope (CS SP8, Leica, Germany).3. Results and discussion
Using the pretreated GO (Fig. S1†), carbon-based FePt nanocomposites were successfully synthesized via our reported methods with moderate modification. Ultrasmall fccFePt NPs are assembled on the GO surface to form FePt/GO nanocomposites. This strategy is schematically illustrated in Scheme 1. First, FePt/GO nanocomposites were synthesized via the simple polyol protocol. Next, the nanocomposites were covalently conjugated with the PEG polymer via EDC/NHS chemistry to endow the NPs with good biocompatibility and physiological stability. Lastly, FA and FITC were covalently bound to the PEG-modified NPs, thus allowing them to specifically target FA receptor-rich cancer cells and giving the capability of simultaneously performing dual-modality imaging by fluorescence and magnetic resonance.Fig. 1a and b show typical TEM images for the as-prepared FePt/GO nanocomposites with different magnifications, showing the uniformly distribution of FePt NPs on the GO surface. More importantly, few aggregations of FePt NPs were found in solution, which indicates the highly selective nucleation of FePt NPs on the functional GO surface. The average diameters of FePt NPs were ca. 2–3 nm. EDX analysis of the samples revealed that the alloying compositions of the three samples were Fe58Pt42. ICP analysis of these samples revealed their final particle composition to be Fe57Pt43, which is consistent with the EDX measurement. The magnetic properties of the as-prepared FePt/GO were determined by field-dependent magnetization measurement. The magnetic hysteresis loops (Fig. 1c) showed that the samples exhibited superparamagnetic behavior at 300 K. The saturated mass magnetizations (Ms) at 300 K are 26.15 emu g−1. Six-arm PEG-amine was then covalently conjugated to the FePt/GO to endow the NPs with good biocompatibility and physiological stability. Conjugation of FA and FITC to FePt/GO–PEG through formation of an amide bond by the reaction between the COOH groups of FA and NH2 groups of FePt/GO–PEG and formation of a thiourea bond by NCS groups of FITC and NH2 groups of FePt/GO–PEG was confirmed by UV/visible and fluorescence measurements. In the UV/visible spectrum (Fig. 1d) of FePt/GO–PEG–FA–FITC, the peak at 230 nm characteristic of GO disappears while a new peak at 278 nm appears due to the presence of FA and FITC in the FePt/GO. A fluorescence peak of FePt/GO–PEG–FA–FITC appears at 518 nm with excitation wavelength of 488 nm, which is consistent with that of free FITC, confirming successful conjugation of FITC on the surface of the FePt/GO NPs. These FePt/GO–PEG–FA–FITC nanocomposites exhibited excellent stability in DMEM with 10% fetal bovine serum for two months without precipitation (Fig. S2†). To elucidate this stability of the NPs in DMEM with 10% fetal bovine serum, the zeta potentials of the FePt/GO–PEG–FA–FITC NPs in water and in DMEM with 10% fetal bovine serum were measured to be −45.9 mV and −15.0 mV, respectively. Clearly, the great difference in the surface charges of the magnetic NPs is due to the zeta potentials of the DMEM with 10% fetal bovine serum without NPs (−13.7 mV), but we still see that the zeta potential of FePt/GO–PEG–FA–FITC NPs in water (−45.9 mV) is lower than −30 mV, which points to the stability of the NPs.
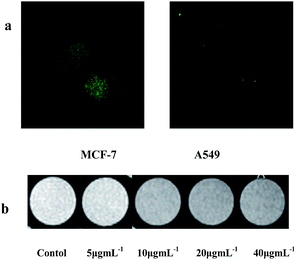
To check the targeting function of the conjugated FA, the complex FePt/GO–PEG–FA–FITC NPs were incubated with MCF-7 cells (FA receptor positive) and A549 cells (FA receptor negative). The cells were imaged by confocal fluorescence microscopy (Fig. 2a). Much stronger fluorescence was observed in MCF-7 cells than in A549 cells for these two samples, indicating higher uptake of FePt/GO–PEG–FA–FITC for MCF-7 cells. This result further demonstrates the high specific folate receptor targeting of FePt/GO–PEG–FA–FITC. Not only can the conjugated FITC be used in fluorescence visualization imaging modalities, but also the attached superparamagnetic FePt NPs are a potential excellent contrast agent for T2-weighted MR images according to the reported literature.11,12 After incubation with the FePt/GO–PEG–FA–FITC NPs, MCF-7 cells were transferred into agarose gel pellets and imaged by 11.7 T Bruker micro scanners. As expected, the NP-labeled MCF-7 cells (Fig. 2b) showed relaxation rate enhancement, depending on the dose. In our study, the imaging contrast for cells incubated with the FePt/GO–PEG–FA–FITC NPs at the Fe concentration of 10 μg mL−1 is obviously darker than that in case without NPs. With the increase of concentration, the growth rate of the MR relaxation rate started to decline. This decrease of growth rate is due to cell apoptosis and cytotoxicity impairment when cells are incubated with the heavily labeled NPs (apoptotic cells can be easily washed). These results indicate the great potential of the as-prepared FePt/GO–PEG–FA–FITC for dual-modal imaging contrast for diagnostic functions. WST cell viability assay was performed to evaluate the cytotoxicity of FePt/GO–PEG–FA–FITC composites to FA receptor-positive MCF-7 cells (Fig. 3a). Experimental data confirmed that the cytotoxicity was dependent on the Fe concentration. The half-maximum inhibitory concentration (IC50) value is 49.65 μg mL−1 for FePt/GO–PEG–FA–FITC. Based on Fig. 2 and 3, one could find that the as-prepared carbon-based nanocomposites exhibit not only dual-modal visualization of cellular uptake by fluorescence and MRI but also potential therapeutic function for FA receptor-positive cancer cells. In our study, MR relaxation rate (1/T2) of MCF-7 cells incubated with the FePt/GO–PEG–FA–FITC nanocomposites at different concentrations for 24 h is still very high (12.7 mM−1 s−1).
It is well known that the uptake of Fe NPs in cellular systems will lead to Fe buildup and the production of ROS. These effects will induce apoptosis and inflammation,8,16,23,24 affect the actin cytoskeleton, damage DNA and proteins25 and eventually lead to cell death. According to the reported results, it is believed that iron ions released after the degradation of NPs into the acidic endolysosomal compartments could react with hydrogen peroxide produced by the mitochondria, producing highly reactive hydroxyl radicals and ferric ions (Fe3+) through the Fenton reaction.16,26,27 Here, the assessment of NPs in inducing the production of intracellular oxidants was characterized by DCF fluorescence as a reporter of ROS generation. The MCF-7 cells were firstly incubated with DCFH-DA for 20 min, and then treated with FePt/GO–PEG–FA–FITC at final Fe concentrations of 20 μg mL−1, 60 μg mL−1, and 100 μg mL−1. Following cellular uptake, the DCFH is deprotonated and converted to quinone-like DCF in the presence of ROS. After 6 h of cells-NPs interaction, the MCF-7 cells were tested by fluorescence spectrophotometry (excited at 488 nm), as shown in Fig. 3b and S3.† The higher the NP concentration (100 μg mL−1), the higher the fluorescence intensity. Obvious toxicity was found at Fe concentrations of 50 μg mL−1 and 100 μg mL−1, which is consistent with the ROS results.
In order to further test the impact of this nanocomposite on DNA, we performed the DNA Ladder Extraction assay of cell apoptosis. The DNA genotoxicity results were in good agreement with the cytotoxicity data: the higher the Fe concentration, the more significant the damage of DNA in terms of DNA fragmentation length and DNA fragmentation percentage (Fig. 4a). This modality of DNA fragmentation is typical of ROS-induced cell death, i.e., giant DNA fragmentation followed by smaller internucleosomal DNA fragmentation shown as a ladder-like pattern.28,29 These data suggest that the NPs induced DNA fragmentation in an ROS-dependent manner. An ultrastructural examination of the cells treated with FePt/GO–PEG–FA–FITC NPs was performed to confirm that the NPs had interacted with the cells. Obviously, the individual FePt/GO–PEG–FA–FITC NPs with diameters of 200 nm by TEM were observed in ultrathin sections (Fig. 4b). Visible morphological damage was observed for the MCF-7 cells treated with the NPs. A few endoplasmic reticulum and small Golgi complex were damaged by the NPs as observed by TEM. Many endosomes and lysosomes were observed which demonstrated the toxicity of these NPs. Iron inside the cells was visualized by Prussian blue staining, a standard protocol widely used to examine cellular uptake of iron oxide NPs.30 We found that the FePt/GO–PEG–FA–FITC NPs labeled cells efficiently, labeled at nearly 90% efficiency (Fig. 4c and d). The labeled cells showed clusters of dense blue granules in the cytoplasm, which indicates the location of the NPs.
4. Conclusions
In this work, we have developed a simple strategy for the preparation of FeP/GO nanocomposites as a therapeutic nanoplatform that combines efficient targeting, dual-modal imaging and therapeutic properties. The conjugation of biocompatible PEG, FA and FITC endows the as-prepared carbon-based nanocomposites with high stability in physiological solutions, targeted delivery to folate receptor-rich cancer cells and dual-modal visualization by fluorescence (FITC) and magnetic resonance imaging. The dual-modal optical/MRI imaging combines the advantages of each single imaging mode to enhance the sensitivity and tissue penetration. After targeting uptake with MCF-7 cells, the as-prepared FePt/GO NPs selectively initiated catalytic formation of ROS, which are toxic to tumor cells. The uptake of Fe into cells and the cytotoxicity mechanism were further characterized by Prussian blue staining, ultrastructural examination and the DNA Ladder Extraction. The as-prepared FePt/GO NPs could in principle be coated and conjugated with various kinds of lipid molecules, peptides and antibodies that are specific for different cancer cells. Therefore, the carbon-based FePt NPs are promising for many applications in biomedicine, including multimodality imaging, cell tracking and imaging-guided novel targeted cancer therapies. Further works are ongoing.Acknowledgements
Financial support of this work by the Natural Science Foundation of China (nos 21101087, 21375057, 21205057, 21405074) and Shandong Province Natural Science Foundation (no. ZR2013BL007) is gratefully acknowledged.Notes and references
- C. Li, Nat. Mater., 2014, 13, 110–115 CrossRef CAS PubMed.
- P. Prabhu and V. Patravale, J. Biomed. Nanotechnol., 2012, 8, 859–882 CrossRef CAS PubMed.
- S. S. Kelkar and T. M. Reineke, Bioconjugate Chem., 2011, 22, 1879–1903 CrossRef CAS PubMed.
- N. Ahmed, H. Fessi and A. Elaissari, Drug Discovery Today, 2012, 17, 928–934 CrossRef CAS PubMed.
- D. Yoo, J. H. Lee, T. H. Shin and J. Cheon, Acc. Chem. Res., 2011, 44, 863–874 CrossRef CAS PubMed.
- K. Yang, L. Z. Feng, X. Z. Shi and Z. Liu, Chem. Soc. Rev., 2013, 42, 530–547 RSC.
- H. T. Ke, J. R. Wang, Z. F. Dai, Y. S. Jin, E. Z. Qu, Z. W. Xing, C. X. Guo, X. L. Yue and J. B. Liu, Angew. Chem., Int. Ed., 2011, 50, 3017–3021 CrossRef CAS PubMed.
- W. Gao, L. F. Ji, L. Li, G. W. Cui, K. H. Xu, P. Li and B. Tang, Biomaterials, 2012, 33, 3710–3718 CrossRef CAS PubMed.
- L. Cheng, K. Yang, Y. G. Li, J. H. Chen, C. Wang, M. W. Shao, S. T. Lee and Z. Liu, Angew. Chem., Int. Ed., 2011, 50, 7385–7390 CrossRef CAS PubMed.
- Y. Park, H. M. Kim, J. H. Kim, K. C. Moon, B. Yoo, K. T. Lee, N. Lee, Y. Choi, W. Park, D. S. Ling, K. Na, W. K. Moon, S. H. Choi, H. S. Park, S. Y. Yoon, Y. D. Suh, S. H. Lee and T. Hyeon, Adv. Mater., 2012, 24, 5755–5761 CrossRef CAS PubMed.
- S. Chen, L. J. Wang, S. L. Duce, S. Brown, S. Lee, A. Melzer, S. A. Cuschieri and P. Andre, J. Am. Chem. Soc., 2010, 132, 15022–15029 CrossRef CAS PubMed.
- S. W. Chou, Y. H. Shau, P. C. Wu, Y. S. Yang, D. B. Shieh and C. C. Chen, J. Am. Chem. Soc., 2010, 132, 13270–13278 CrossRef CAS PubMed.
- T. Fuchigami, R. Kawamura, Y. Kitamoto, M. Nakagawa and Y. Namiki, Biomaterials, 2012, 33, 1682–1687 CrossRef CAS PubMed.
- Y. M. Liu, K. Yang, L. Chen, J. Zhu, X. X. Ma, H. Xu, Y. G. Li, L. Guo, H. W. Gu and Z. Liu, J. Nanomed. Nanotechnol., 2013, 9, 1077–1088 CrossRef CAS PubMed.
- N. Moghimi and K. T. Leung, Anal. Chem., 2013, 85, 5974–5980 CrossRef CAS PubMed.
- C. J. Xu, Z. L. Yuan, N. Kohler, J. M. Kim, M. A. Chung and S. H. Sun, J. Am. Chem. Soc., 2009, 131, 15346–15351 CrossRef CAS PubMed.
- S. Maenosono and S. Saita, IEEE Trans. Magn., 2006, 42, 1638–1642 CrossRef CAS.
- C. L. Chen, L. R. Kuo, S. Y. Lee, Y. K. Hwu, S. W. Chou, C. C. Chen, F. H. Chang, K. H. Lin, D. H. T. sai and Y. Y. Chen, Biomaterials, 2013, 34, 1128–1134 CrossRef CAS PubMed.
- K. Yang, L. Z. Feng, X. Z. Shi and Z. Liu, Chem. Soc. Rev., 2013, 142, 530–547 RSC.
- K. Yang, L. Z. Feng, H. Hong, W. B. Cai and Z. Liu, Nat. Protoc., 2013, 8, 2391–2403 Search PubMed.
- L. M. Zhang, J. G. Xia, Q. H. Zhao, L. W. Liu and Z. J. Zhang, Small, 2010, 6, 537–544 CrossRef CAS PubMed.
- Q. T. Hu, Z. B. Gan, X. W. Zheng, A. H. Zhao and X. Zhang, J. Nanopart. Res., 2011, 13, 3191–3197 CrossRef CAS.
- M. A. Malvindi, V. De Matteis, A. Galeone, V. Brunetti, G. C. Anyfantis, A. Athanassiou, R. Cingolani and P. P. Pompa, PLoS One, 2014, 9, e85835 Search PubMed.
- A. Laskar, M. Ghosh, S. I. Khattak, W. Li and X.-M. Yuan, Nanomedicine, 2012, 7, 705–717 CrossRef CAS PubMed.
- N. Singh, G. J. S. Jenkins, B. C. Nelson, B. J. Marquis, T. G. G. Maffeis, A. P. Brown, P. M. Williams, C. J. Wright and S. H. Doak, Biomaterials, 2012, 33, 163–170 CrossRef CAS PubMed.
- M. Levy, F. Lagarde, V. A. Maraloiu, M. G. Blanchin, F. Gendron, C. Wilhelm and F. Gazeau, Nanotechnology, 2010, 21, 395103 CrossRef PubMed.
- S. J. H. Soenen, U. Himmelreich, N. Nuytten and M. De Cuyper, Biomaterials, 2011, 32, 195–205 CrossRef CAS PubMed.
- K. Takaki, Y. Higuchi, M. Hashii, C. Ogino and N. Shimizu, J. Biosci. Bioeng., 2014, 117, 129–133 CrossRef CAS PubMed.
- Y. Higuchi, Biochem. Pharmacol., 2003, 66, 1527–1535 CrossRef CAS.
- A. S. Arbab, L. A. Bashaw, B. R. Miller, E. K. Jordan, B. K. Lewis, H. Kalish and J. A. Frank, Radiology, 2003, 229, 838–846 CrossRef PubMed.
- Z. Liu and X. J. Liang, Theranostics, 2012, 2, 235–237 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: AFM image for GO, TEM image for FePt/CNTs, etc. See DOI: 10.1039/c4ra11589a |
| This journal is © The Royal Society of Chemistry 2014 |