The chemistry and biological activities of natural products from Northern African plant families: from Aloaceae to Cupressaceae
Fidele Ntie-Kang†
*ab and
Joseph N. Yong†
*b
aDepartment of Chemistry, Chemical and Bioactivity Information Centre, Faculty of Science, University of Buea, P.O. Box 63, Buea, Cameroon. E-mail: ntiekfidele@gmail.com; fidele.ntie-kang@ubuea.cm; Tel: +237 77915473
bDepartment of Chemistry, Faculty of Science, University of Buea, P.O. Box 63, Buea, Cameroon. E-mail: joseph.yong@ubuea.cm
First published on 7th November 2014
Abstract
Traditional medicinal practices play a key role in health care systems in countries with developing economies. The aim of this survey was to validate the use of traditional medicine within Northern African communities. In this review, we summarize the ethnobotanical uses of selected plant species from the Northern African flora and attempt to correlate the activities of the isolated bioactive principles with known local uses of the plant species in traditional medicine. The literature is covered for the period 1971 to 2014. Part I of this series focuses on plant families with names beginning with letters A to C, with the ethnobotanical uses of 32 plant species correlated with the bioactivities of 176 compounds identified.
1 Introduction
Before the advent of modern or Western medicine, people from several civilizations have depended on medicinal plants/herbs for the treatment of various diseases and ailments. This is particularly the case in the Northern African civilization of Egypt.1 Ancient Egyptians are known to have been familiar with a catalogue of plant-derived traditional remedies, having used a range of plant organs; roots, rhizomes, flowers, leaves, fruits, seeds, and oils in the form of powders, pills, suppositories, creams, pastes, and ointments.2,3 In modern Egyptian society, plant-derived remedies are popularly sold in herbal shops and used by the local populations, often without the need for scientific evidence for their use.1 Some of the knowledge derived from the Ancient civilization of Egypt has been transmitted throughout North Africa and the Middle East.According to the United Nations Organization (UNO), the geographical region of Northern Africa comprises the countries; Algeria, Egypt, Libya, Morocco, North Sudan, South Sudan, Tunisia and Western Sahara (Fig. 1), covering a land surface area of about 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 935
935![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 659 km2 and a total population of 198
659 km2 and a total population of 198![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 996
996![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 inhabitants.4 The inhabitants of North Africa are generally divided in a manner roughly corresponding to the principal geographic regions of North Africa: the Maghreb, the Nile Valley, and the Sahara. This region is mostly desert (the Sahara), while the rest is made of oases and grasslands, with a few species of trees.5 According to the analyses of Vilà et al., a total of 343 vascular plant species from 69 families non-native to this region were found in the literature.5 The authors mentioned that alien species richness ranged from 143 (Algeria) to 60 (Tunisia). Most of these were of Mediterranean and North American origin.5 For the purposes of this study, the terminology “North Africa” is slightly different from that defined by the World Geographical Scheme for Recording Plant Distributions,6 since the latter excludes the countries of Northern and Southern Sudan.
526 inhabitants.4 The inhabitants of North Africa are generally divided in a manner roughly corresponding to the principal geographic regions of North Africa: the Maghreb, the Nile Valley, and the Sahara. This region is mostly desert (the Sahara), while the rest is made of oases and grasslands, with a few species of trees.5 According to the analyses of Vilà et al., a total of 343 vascular plant species from 69 families non-native to this region were found in the literature.5 The authors mentioned that alien species richness ranged from 143 (Algeria) to 60 (Tunisia). Most of these were of Mediterranean and North American origin.5 For the purposes of this study, the terminology “North Africa” is slightly different from that defined by the World Geographical Scheme for Recording Plant Distributions,6 since the latter excludes the countries of Northern and Southern Sudan.
Recently, our research team has started to explore the components of African traditional medicines, by documenting the current available knowledge on the biological activities of natural products (NPs) isolated from the African flora (from dispersed data/literature sources), to aid in drug discovery programs on the continent. This includes the development of NP databases for use in virtual screening campaigns,7–10 the pharmacokinetic profiling of NP databases from African flora,10–13 developing focused reviews on NPs isolated from floral matter from particular countries/regions,14–19 as well as NPs with interesting biological activities against specific diseases/ailments.20–23 Our focus has been on the NPs whose measured biological activities correlate with the use of the plant in African traditional medicine (ATM), with the aim of validating the use of these plant species in traditional medicine. In this survey, the ethnobotanical uses of the plant species from Northern Africa versus the bioactivities of the derived NPs are presented by plant family and in alphabetical order. Wherever the bioactivities of the isolated metabolites correlate with the ethnobotanical uses of the plant species, these are highlighted in bold in the tables. In the first part of this review series, only plant families with names beginning with letters A to C have been considered.
2 Aloaceae (now Xanthorrhoeaceae-Asphodeloideae), Anacardiaceae and Apiaceae
The traditional uses of the studied species from the above families, as well as the isolated compounds and their respective bioactivities are shown in Table 1, while the chemical structures are shown in Fig. 2 and 3. The species from the Aloaceae; Aloe vera24 and Aloe sinkatana25 from North Africa have been investigated. A. vera is a well known medicinal plant with diverse applications, in horticulture, medicine and commerce.24–27 Milardi et al. investigated the antioxidant properties of the ethanolic extract of A. vera leaf skin harvested in Kairouan, Tunisia.24 This was fractionated by liquid–liquid partition using hexane, ethyl acetate, chloroform–ethanol and butanol. The chloroform–ethanol fraction showed the highest proportion of total phenolics (40.500 ± 0.041 μg gallic acid equivalents per g of extract), the highest scavenging activity and the greatest reducing power, followed by ethyl acetate, butanol and hexane extracts. ELhassan et al. also investigated the leaves of Aloe sinkatana, collected from Arkawit, Sudan. In Sudanese traditional medicine, the leaves and leaf exudates of this plant are valued to treat a variety of ailments, including skin disease, constipation, fever and inflamed colon,28 as well as in the treatment of diabetes.29 The phytochemical investigation of this plant led to the identification of a new anthraquinone, 2,8-dihydroxy-6-(hydroxymethyl)-1-methoxyanthracene-9,10-dione (1), along with 10 known compounds.25 The known compounds include; aloe-emodin (2), feralolide (3), 1-hydroxy-5-methoxy-3-methyl-9,10 dihydroanthracene 9,10-dione (4), β-sitosterol (5), β-sitosterol with glycosidic bond (6), microdontin (7), homoaloins A (8) and B (9) and aloins A (10) and B (11). Scientific evidence for the use of this plant in the treatment of diabetes lies in the fact that compound 1 also showed significant inhibitory effects against glucose-induced advanced glycation end-products.25| Plant family | Plant name (country) | Use in traditional medicine | Part of plant studied | Active principle | Measured activity | Author and Reference |
|---|---|---|---|---|---|---|
| Aloaceae (now Xanthorrhoeaceae-Asphodeloideae) | Aloe vera and Aloe sinkatana (Tunisia, Sudan) | Well known medicinal plants with diverse applications, in horticulture, medicine and commerce | Whole plant | 1–11 | Inhibitory effects against glucose-induced advanced glycation end-products | Miladi et al.,24 ELhassan et al.25 |
| Anacardiaceae | Schinus molle (Saudi Arabia) | Traditionally used as a repellent against house flies, Musca domestica | Fruits and leaves | 12 | Insect repellent activity | Abdel-Sattar et al.30 |
| Apiaceae | Daucus carota carota(Tunisia) | Used traditionally for the treatment of hepatic and renal insufficiency as well as for skin disorders | Roots | 13–19 | Antifungal activity | Ahmed et al.35 |
| Torilis radiata (Egypt) | Hepatoprotective | Aerial parts | 26–32 | Diverse biological activities | Ezzat et al.39 | |
| Pituranthos scoparius (Algeria) | Used in traditional medicine in the treatment of asthma and rheumatism and in food as flavouring. | Aerial parts | 33 and 34 | Antioxidant, antibacterial, anticancer, antihistamic, and other biological effects | Dahia et al.68 |
From the Anacardiaceae (cashew or sumac family), foliage from the pepper tree, Schinus molle, is traditionally used as a repellent against house flies, Musca domestica, in Ethiopia.30 It is also valuable as a purgative, diuretic, parasiticide and vulnerary.31 The repellent ability of the ethanol and petroleum ether extracts from the leaves and fruits of S. molle against adult Blattella germanica have been investigated,32 while hexane extracts from the leaves and the fruits have been tested for repellent and insecticidal activities against nymphs and eggs of the Chagas' disease vector, Triatoma infestans.33 Abdel-Sattar et al. have investigated the insecticidal and insect repellent activities of essential oils from the leaves and fruits of the plant species S. molle, harvested in Saudi Arabia and attempted to establish a correlation with the chemical compositions of these oils documented.30 GC-MS analysis led to the identification of sixty five (65) components, showing that hydrocarbons dominated the oil composition, with monoterpenes occurring in the largest amounts in the fruits and leaves, 80.43% and 74.84%, respectively. Additionally, p-cymene (12) was identified as a major component in both oils. It could be concluded that the high yield and efficacy of S. molle essential oil against T. granarium and T. castaneum suggest that the oil may provide leads for active insecticidal agents.30
Daucus carota carota (Apiaceae: celery, carrot or parsley family) is native to the continents of Europe, Asia and Africa. Extracts of the plant are used traditionally for the treatment of hepatic and renal insufficiency as well as for skin disorders.34 Extracts of the wild plants are also known to exhibit antioxidative and iron-chelative properties. Ahmed et al. have isolated three new sesquiterpene daucane derivatives (13–15) and four known compounds (16–19) from the roots of wild D. carota carota, that showed antifungal activity.35 The methanol extract of the seeds of this plant also showed antibacterial activity.36 Marzouli et al. investigated essential oils and supercritical CO2 extracts of umbels from wild Daucus carota carota from two different sites in Tunisia.37 It was shown that eudesm-7(11)-en-4-ol (20) (8.2–8.5%), carotol (21) (3.5–5.2%), sabinene (22) (12.0–14.5%), α-selinene (23) (7.4–8.6%) and 11-α-(H)-himachal-4-en-1-β-ol (24) (12.7–17.4%) were dominant in the oil from the species harvested in Sejnane, whereas the oils from Tunis were predominantly composed of elemicin (25) (31.5–35.3%) and carotol (21) (48.0–55.7%). The antimicrobial activity of the essential oils were assayed by using the broth dilution method on Escherichia coli ATCC 35218 and Staphylococcus aureus ATCC 43300, and clinical strains of Candida albicans and C. tropicalis 1011 RM. The MIC values obtained were all >2.5% (v/v).
Traditional plant remedies have been extensively evaluated for their antihepatotoxic activity through in vivo and in vitro test models (since effective treatments against liver disease are rare). Ozbek et al. have presented an extensive report on the hepatoprotective efficacy of certain plants from the Apiaceae.38 This has warranted some investigation of apiaceous species from Egypt.39 The hepatoprotection of the ethanolic extract and fractions of the aerial parts of Torilis radiata (Apiaceae), from Egypt, were assessed in terms of the reduction in histological damage, accompanied by restoration of the liver enzymes; alanine amino transferase, aspartate amino transferase, lactate dehydrogenase, and a reduction in the inflammatory markers (tumour necrosis-α, nitric oxide, N-acetyl-β-D-glucosaminidase and myloperoxidase) in serum.39 An investigation of the aqueous ethanolic extract of the aerial parts of the plant yielded two triterpenes; lupeol acetate (26) and α-amyrin (27), along with spinasterol (28) from the n-hexane fraction; while acacetin (29), scopoletin (30) and ferulic acid (31) were derived from the chloroform fraction and luteolin-7-O-glucoside (32) from the n-butanol fraction.39 The isolated compounds have exhibited a number of bioactivities. Lupeol acetate, for example, is known to exhibit anti-inflammatory40 and antiarthritic41 activities, while α-amyrin has shown anti-inflammatory,42,43 antihyperglycemic44 and antimicrobial45 activities, along with antinociceptive properties in mixture with β-amyrin.46 In vivo studies clearly demonstrated that spinasterol exhibits antitumorigenic activity against skin tumors with neither co-tumour nor co-carcinogen promoter activities.47 The compound exhibits its cytoprotective and anti-inflammatory effects via the induction of heme oxygenase-1 in murine hippocampal and microglial cell lines.48 Acacetin is known to inhibit cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells,49 along with other cancer types.50–53 Scopoletin exhibits a host of biological activities, including acetylcholinesterase inhibitory activity,54 hepatoprotective activity,55 xanthine oxidase inhibition and uricosuric activities56 and antimicrobial activities.57,58 Ferulic acid and some of its derivatives are known to exhibit antioxidant59,60 and hypoglycaemic60,61 activities, among other activities,62 while luteolin-7-O-glucoside displayed antiasthmatic,63 antioxidant,64 antiviral,65,66 and antibacterial properties.67 Thus, the hepatoprotective activity of scopoletin could justify the use of this apiaceous plant in the treatment of liver disorders among other ailments.
Dahia et al. also investigated the methanolic and aqueous extracts of Pituranthos scoparius, another apiaceous medicinal plant harvested from Southern Algeria.68 Fifteen metabolites were identified, including two cinnamic acids; 5-O-caffeoyl quinic acid (33) and 5-feruloyl quinic acid (34), along with thirteen known flavonoids, including vicenin-2 (35), six quercetin and six isorhamnetin O-glycosylated derivatives. Among the isolated metabolites, 5-O-caffeoyl quinic acid was the main component, while, of the flavonoids, the isorhamnetin derivatives were present to a greater extent. The major component of this plant (compound 33) is known to exhibit several bioactivities, including antioxidant, antibacterial, anticancer, antihistamic, and other biological effects.69
3 Arecaceae, Asclepiadaceae (now Apocynaceae-Asclepiadoideae) and Asteraceae
The medicinal uses of plants in these plant families are shown in Table 2. Also indicated in the table are the NPs from different plant species with some biological activity which can be correlated with ethnobotanical studies. The chemical structures of the isolated metabolites have been shown in Fig. 4–6.| Plant family | Plant name (Country) | Use in traditional medicine | Part of plant studied | Active principle | Measured activity | Author and Reference |
|---|---|---|---|---|---|---|
| Arecaceae | Livistona australis (Egypt) | To treat tumors | Leaves | 36 | Antioxidant, cytotoxic | Kassem et al.70 |
| Asclepiadaceae | Caloptropis procera (Egypt) | Indigestion, stomach pains, tumors, snake bites, malaria, skin diseases, abdominal viscera, intestinal worms, dyspepsia | Vegetative stems | 51–56, 57–60 | Cytotoxicity, anticancer | Shaker et al.,71 Ibrahim et al.75 |
| Pergularia tomentosa (Algeria) | Skin diseases, bronchitis, tuberculosis, abortifacient, worms, scorpion bites, stomach pains, used as arrow poisons, used for tanning | Whole plant | 66 and 67 | None | Babaamer et al.76 | |
| Asteraceae | Artemisia herba-alba(Algeria) | Tea preparations with components of this species have exhibited a number of therapeutic properties, including analgesic, antibacterial, anthelminthic and diuretic | Whole plant | 68–83 | Not determined | Laid77 |
| Chiliadenus montanus (Egypt) | Renal problems, used as herbal tea | Aerial parts | 84–91 | Antimicrobial | Hegazy et al.78 | |
| Chrysanthemum macrocarpum (Algeria) | Inflammation, headache, ulcerative colitis, vertigo, eye irritation, hypertension, intestinal infections, scabies | Aerial parts | 103, 106 | Antimicrobial, anticancer | Boutaghane et al.83 | |
| Gaillardia aristata(Egypt) | Used as a diuretic, to relieve painful urination | Leaves | 76, 77 | Cytotoxicity | Salama et al.87 | |
| Wedelia prostrata (Egypt) | Kaurene diterpenes have shown antibiotic activity | Fresh young shoots | Not tested | Ahmed et al.88 | ||
| Conyza bonariensis (Tunisia) | Inflammation, vermifuge, diarrhoea, haemorrhoids, used as a diuretic | Leaves, stem, roots | Not tested | Mabrouk et al.89 | ||
| Centaurea nicaeensis (Algeria) | Aerial parts | 120 | Not tested | Hammoud et al.90 | ||
| Psiadia punctulata (Saudi Arabia) | Used in casts for broken bones | Leaves and stems | Abou-Zaid et al.91 |
From the Arecaceae (palm tree family), a number of compounds of medicinal value were isolated and screened for biological activities against various diseases. A new flavone glycoside, tricin 7-O-β-glucopyranoside-2′′-sulphate sodium salt (36) and fourteen known flavonoids (37–50) were isolated from the leaves of Livistona australis and screened for antioxidant and cytotoxicity properties.70 Compound 36 and the methanol extract of the leaves of L. australis significantly restored the reduced levels of GSH in diabetic rats. Compound 36 also indicated potent cytotoxic activity against liver carcinoma Hepg2, breast carcinoma MCF7 and colon carcinoma HCT116 cell lines, with IC50 values of 13.5, 15.2 and 16.5 μg mL−1, respectively. The ethanol extract showed less activity against HEPG2 and MCF7 cell lines with IC50 values of 21.9 and 22 μg mL−1 respectively, and the weakest activity against colon carcinoma HCT116 cell line, with IC50 values of 45.8 μg mL−1.71 The known compounds isolated, alongside compound 36 are genkwanin-6-C-β-glucopyranoside (37), genkwanin 8-C-β-glucopyranoside (38), isovitexin (39), isoorientin (40), orientin (41) tricin 7-O-β-glucopyranoside (42), tricin 4′-O-β-glucopyranoside (43), luteolin 7-O-β-glucopyranoside (44), quercetin 3-O-β-glucopyranoside (45), qurecetin 3-O-β-galactopyanoside (46), tricin (47), quercetin (48), apigenin (49) and luteolin (50).
Calotropis procera (Aiton) (Asclepiadaceae) is a traditional medicinal plant that has been used for the treatment of various ailments in Africa and Asia. The root bark is used in treating skin diseases, enlargement of the abdominal viscera, intestinal worms and ascites. The root has also been used as a carminative in the treatment of indigestion. The latex of the plant is used in India as a purgative and the flowers as a digestive and has also shown anti-asthmatic properties.72 An aqueous extract of the latex inhibits cellular infiltration as well as protecting against the development of neoplastic changes in the transgenic mouse model of hepatocellular carcinoma.73 The chloroform extract of the root has been shown to have protective activity against carbon tetrachloride-induced liver damage.74 The ethanol extract of fresh vegetative parts of Calotropis procera from Egypt afforded three new metabolites: 5-hydroxy-3,7-dimethoxyflavone-4′-O-β-glucopyranoside (51), 2β,19-epoxy-3β,14β-dihydroxy-19-methoxy-5α-card-20(22)-enolide (54) and β-anhydroepidigitoxigenin-3β-O-glucopyranoside (55), along with two known compounds, uzarigenine (52) and β-anhydroepidigitoxigenin (53).71 Compound 56 was used as a reference for structural analysis. Ibrahim et al. also isolated four new ursane-type triterpenes, namely, calotroprocerol A (57), calotroproceryl acetate A (58), calotroprocerone A (59) and calotroproceryl acetate B (60) from the root bark of C. procera along with five known compounds: pseudotaraxasterol acetate (61), taraxasterol (62), calotropursenyl acetate B (63), stigmasterol (64) and (E)-octadec-7-enoic acid (65).75 The bioactivities of the newly identified metabolites are still to be determined.
Compounds 51–55 were tested in vitro for cytotoxicity against the human cells HT29 and Hepg2 and the mouse fibroblast cell line NIH-3T3. Uzarigenine (52) decreased the metabolic activity of HT29 and HEPG2 cells at 50 μM concentration by 59% and 35%, respectively, and no decrease of the metabolic activity of the NIH-3T3 cells was noted. Compound 51 (50 μM) decreased the metabolic activity of NIH-3T3 and HEPG2 cells by 18% and 10%, respectively, but compounds 52, 53 and 55 indicated no activity. The metabolites displayed no significant antimicrobial activity against a panel of bacterial strains.71 Compounds 57–65 were all evaluated against three human cancer cell lines including the A549 non-small cell lung cancer (NSCLC), the U373 glioblastoma (GBM) and the PC3 prostate cancer lines. Only compound 57 showed in vitro growth inhibitory activity in all three cancer cell lines. Compounds 59 and 64 indicated weak in vitro inhibition in A549 NSCLC cancer cell only. The presence of the OH group in compound 57 may be responsible for the in vitro growth inhibition of cancer cells as this property is lost when the OH is protected or oxidized (as opposed to compounds 58, 60, 61 and 63).
Two new taraxastane-type triterpenes (66 and 67), along with eight known compounds (68–75) were isolated from Pergularia tomentosa (Asclepiadaceae) from Algeria.76 The new compounds were named pergularine A (66) and pergularine B (67). The known compounds were oleic acid (68), (9Z,12Z)-octadecadienoic acid (69), α-amyrin (70), 3-acetyltaraxasterol (71), 3-taraxasterol (72), 16α-hydroxytaraxasterol-3-acetate (73), 3-epimicromeric acid (74) and (9Z,12Z)-octadecadienoic acid glucoside (75). Although this plant is poisonous, it is used in folk medicine as a tanning agent and for the treatment of skin diseases in Nigeria. It is used as a depilatory, a poultice, a laxative, an anthelmintic and an abortifacient in Egypt. A decoction of the leaves and stem is used as a remedy for bronchitis and tuberculosis as well as a molluscicide. Unfortunately, the new compounds were not tested for any biological activities.
A number of medicinal plants in the Asteraceae (aster, daisy, composite, or sunflower family) have been investigated in North Africa, mostly for their antimicrobial and anticancer activities.77–80 From Artemisia herba-alba, harvested from Eastern Algeria, Laid isolated six compounds, comprising two new sesquiterpene lactones from the methylene chloride/methanol extract of the aerial parts; 1b,9b-diacetoxyeudesm-3-en-5a,6b,11bH-12,6-olide (76) and 1b,9b-diacetoxyeudesm-4-en-6b,11bH-12,6-olide (77), together with 4 known compounds (78–81).77 The species A. herba-alba is famous plant used tremendously in our folk medicine. Tea preparations with components of this species have exhibited a number of therapeutic properties, including analgesic, antibacterial, antihelminthic and diuretic.77 Among the popular compounds from this plant are the terpenoid thujone (82)81 and the flavonoid hispidulin (83).82 However, no correlation between the bioactivities of the isolated metabolites and the ethnobotanical of this plant has been recorded.
Hegazy et al. isolated and characterized eight new (84–91) and eight known metabolites (92–99) from the CH2Cl2/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) extract of the aerial parts of Chiliadenus montanus.78 In Egypt, this plant is known locally as “haneida” and it used as a herbal tea for the treatment of renal problems and some of its constituents have shown anti-diabetic, antimicrobial, anti-obesity, antiatherogenic and antioxidant properties.79,80 The new compounds include 3-oxo-γ-costic acid β-D-glucopyranoside (84), 3β-methoxy isocostic acid (85), 3α-methoxy isocostic acid (86), eudesm-11,13-ene-1β,4β,7α-triol (87), chiliadenol A (3,6,7-trihydroxy-11-methoxy-3,7,11-trimethyldodeca-1,9-diene) (88), chiliadenol B (3-hydroxy-3,7,11-trimethyl-1,6-dodecadien-9-one) (89), chiliadenol C (3-hydroxy-3,11-dimethyl-6β,9α-epidioxy-dodeca-1,7(14),10-triene) (90), chiliadenol D (3-hydroxy-3,11-dimethyl-6β,9α-epidioxy-dodeca-1,10,7(14)-ene (91) and the known ones include; 3-oxo-γ-costic acid (92), 3-oxo-γ-costic acid methyl ester (93), 3α-acetyl-γ-costic acid (94), 5α-hydroxy-4α,15-dihydrocostic acid (95), 5α-hydroxycostic acid (96), eudesmane-1β,4β,7α-triol (97), kaempferol-3-O-(6′′-O-acetyl)-β-D-glucopyranoside (98), and thymol-β-D-glucopyranoside (99). All these isolates were tested for antimicrobial activity against gram-positive bacterial strains, for Staphylococcus aureus and Bacillus subtilis and the gram-negative bacterial strains, Klebsiella pneumoniae, Alcaligenes faecalis and Escherichia coli. The fungal yeasts used were Saccharomyces cerevisiae and Candida albicans. Compound 98 indicated antimicrobial activity preventing the growth of Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumoniae, Alcaligenes faecalis and Candida albicans with MIC values 25, 25, 25, 12.5 and 3.125 μg mL−1, respectively, using chloramphenicol (<6 μg mL−1) as positive control.
1) extract of the aerial parts of Chiliadenus montanus.78 In Egypt, this plant is known locally as “haneida” and it used as a herbal tea for the treatment of renal problems and some of its constituents have shown anti-diabetic, antimicrobial, anti-obesity, antiatherogenic and antioxidant properties.79,80 The new compounds include 3-oxo-γ-costic acid β-D-glucopyranoside (84), 3β-methoxy isocostic acid (85), 3α-methoxy isocostic acid (86), eudesm-11,13-ene-1β,4β,7α-triol (87), chiliadenol A (3,6,7-trihydroxy-11-methoxy-3,7,11-trimethyldodeca-1,9-diene) (88), chiliadenol B (3-hydroxy-3,7,11-trimethyl-1,6-dodecadien-9-one) (89), chiliadenol C (3-hydroxy-3,11-dimethyl-6β,9α-epidioxy-dodeca-1,7(14),10-triene) (90), chiliadenol D (3-hydroxy-3,11-dimethyl-6β,9α-epidioxy-dodeca-1,10,7(14)-ene (91) and the known ones include; 3-oxo-γ-costic acid (92), 3-oxo-γ-costic acid methyl ester (93), 3α-acetyl-γ-costic acid (94), 5α-hydroxy-4α,15-dihydrocostic acid (95), 5α-hydroxycostic acid (96), eudesmane-1β,4β,7α-triol (97), kaempferol-3-O-(6′′-O-acetyl)-β-D-glucopyranoside (98), and thymol-β-D-glucopyranoside (99). All these isolates were tested for antimicrobial activity against gram-positive bacterial strains, for Staphylococcus aureus and Bacillus subtilis and the gram-negative bacterial strains, Klebsiella pneumoniae, Alcaligenes faecalis and Escherichia coli. The fungal yeasts used were Saccharomyces cerevisiae and Candida albicans. Compound 98 indicated antimicrobial activity preventing the growth of Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumoniae, Alcaligenes faecalis and Candida albicans with MIC values 25, 25, 25, 12.5 and 3.125 μg mL−1, respectively, using chloramphenicol (<6 μg mL−1) as positive control.
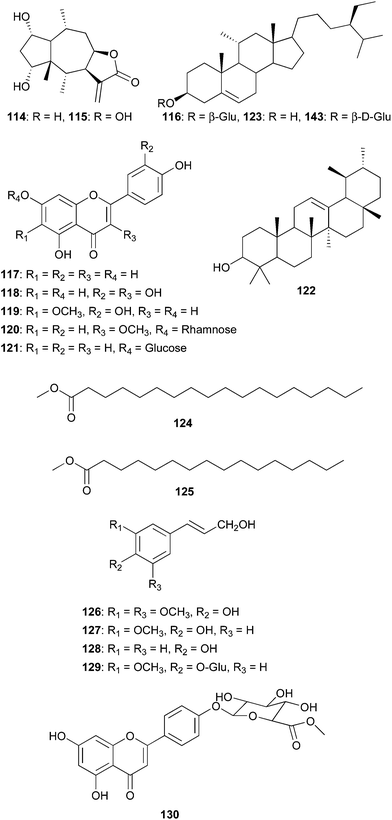
Chrysanthemum macrocarpum (Asteraceae) is an endemic species, which is used in traditional medicine as a scabicide and for the treatment of intestinal infections in Algeria. It is also used in food flavoring and as a herbal tea by the Touaregs.83–86 From the aerial parts of this plant were isolated and characterized three new metabolites: a triterpenic diester (100) and two natural cyclitols, conduritol C (101) and viburnitol (102), in addition to four known triterpenes (103–106) and seven known flavonoids (107–113). Compound 100 was identified as 3,21-dipalmitoyloxy-16β,21α-dihydroxy-β-amyrine and compounds 101 and 102 were determined to be cyclohex-5-ene 1β,2β,3β,4α-tetraol (conduritol C) and cyclohexa-1β,2α,3β,4α,5α-pentaol (viburnitol), respectively. The chloroform, EtOAc and n-BuOH extracts and compounds 103 and 106 were screened for antibacterial activity using two gram-positive bacterial strains (S. aureus and Enterococcus faecalis) and gram negative bacterial strains (Pseudomonas aeruginosa, E. coli and K. pneumoniae) using ampicillin as the standard. Compound 103 (taraxasterol) showed weak antibacterial activity, while compound 106 showed no activity on either bacterial strain. However, the chloroform fraction showed good activity against gram negative bacteria (P. aeruginosa, K. pneumoniae, E. coli.), with MICs of 0.5, 4 and 8 μg mL−1, respectively. Both compound 103 and the chloroform extract were evaluated for their cytotoxic activity against human colon cancer HT-29 cells and human prostate cancer carcinoma PC3 cells. The chloroform fraction and taraxasterol prevented cell proliferation of both HT-29 and PC3 cancer cells in a dose-dependent manner. It was concluded that taraxasterol may be responsible for the activity of the extract since it is known to possess antiproliferative properties against various cancer cells.86 The search for cytotoxic compounds has also led to the bioactivity-guided isolation of ten known compounds from the leaves of Gaillardia aristata (Asteraceae). The compounds are, neopulchellin (114), 6α-hydroxyneopulchellin (115), β-sitosterol-3-O-β-D-glucoside (116), apigenin (117), quercetin (118), eupafolin (119), kaempferol-3-methoxy-7-O-α-L-rhamnoside (120), apigenin-7-O-β-D-glucopyranoside (121), α-amyrin (122) and β-sitosterol (123).83 The antiproliferative activity of these compounds was evaluated against two human cell lines (breast (MCF7) and colon (HCT116)). Compounds 114 and 115 isolated from the chloroform extract indicated the highest cytotoxicity with IC50 values of 0.43, 0.32 μg mL−1 against MCF7 and 0.46, 0.34 μg mL−1 against HCT116, respectively. Compounds 122 and 123 isolated from the hexane extract showed lower IC50 values of 3.05, 2.35 μg mL−1 against MCF7 and 2.9, 2.85 μg mL−1 against HCT116, respectively. The compounds obtained from the EtOAc extract indicated the lowest cytotoxicity.
The ornamental medicinal plant, Wedelia prostrata (Asteraceae) has yielded known compounds from its callus cultures.87,88 The compounds consist of two methyl esters of fatty acids, namely, methyl stearate (124), methyl palmitate (125); four cinnamyl alcohol derivatives, namely sinapyl alcohol (126), coniferyl alcohol (127), p-coumaryl alcohol (128) and coniferyl alcohol 4-O-glucoside (129). These compounds were not tested for any biological activity. Meanwhile, essential oils have been extracted from Conyza bonariensis (Asteraceae), an annual perennial weed endemic in Tunisia.89 The ethanol extract of the aerial parts of Centaurea nicaeensis All. var. walliana M. (Asteraceae), yielded a new flavone glucoside, apigenin 4′-(6′′-methylglucoside (130)), together with six known compounds, namely, cirsilineol, jaceosidin, melitensin, apigenin, apigenin 7-(6′′-methylglucuronide) and prunasin.90 Mamdouh et al. also isolated and characterized fifteen known flavonoids from the leaves of Psiadia punctulata (Asteraceae), but the isolated compounds were not tested for any biological activity.91 The compounds include apigenin, acacetin, luteolin, chrysoeriol, luteolin 7-glucoside, orientin 7-glucoside, isoorientin, isoorientin 7-glucoside, cirsilineol 5,4′-dihydroxy-6,7,3′-trimethoxyflavone, gardenin B (5-hydroxy-6,7,8,4′-tetramethoxyflavone), 5-hydroxy-3,6,7,8,4′-tetramethoxyflavone, 5,3′-dihydroxy-6,7,4′,5′-tetramethoxyflavone, 5-hydroxy-6,7,3′,4′,5′-pentamethoxyflavone, and gardenin C (5,3′-dihydroxy-6,7,8,4′,5′-pentamethoxyflavone).
4 Balanitaceae, Bignoniaceae, Bombacaceae (now Malvaceae-Bombacoideae) and Burseraceae
A summary of the medicinal uses and biological activities of the compounds of the plant families are indicated in Table 3. The chemical structures of the isolated compounds are shown in Fig. 7.| Plant family | Plant name | Use in traditional medicine | Part of plant studied | Active principle | Measured activity | Author and Reference |
|---|---|---|---|---|---|---|
| Balanitaceae | Balanites aegyptiaca (Mali) | Treatment of jaundice and diabetes | Stem bark | 131, 132 | Not tested | Sarker et al.92 |
| Bignoniaceae | Tabebuia argentea (Mali) | Inflammation, influenza | Leaves | 131 | Not tested | de Abreu et al.93 |
| Catalpa bignonioides (Egypt) | Whooping cough, asthma, asthmatic cough | Petioles | 132 | Not tested | de Abreu et al.93 | |
| Jacaranda mimosaefolia (Egypt) | Anti-malarial, antioxidant, anti-inflammatory, antisyphilitic astringent; veneral diseases | Stem bark | 135–137 | Not tested | Zaghloul et al.95 | |
| Bombacaceae (now Malvaceae-Bombacoideae) | Bombax malabaricum (Egypt) | Diuretic, demulcent, aphrodisiac, emetic; importance, enteritis, dysentery, lymphadenoma, menorrhagia, hepatitis | Flowers | n-Hexane, methanol extracts | Antioxidant, antimicrobial | El-Hagrassi et al.100 |
| Burseraceae | Commiphora molmol (North Africa) | Insect repellent, antiseptic, anti-inflammatory; mouth and throat infections including gingivitis, tonsillitis and mouth ulcers. | Bark | 151–154, extract | Antibacterial | Rahman et al.88 |
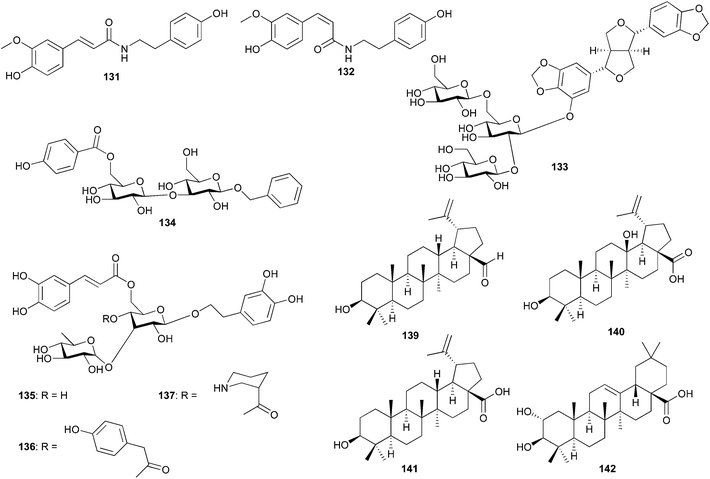
Balanites aegyptiaca (Balanitaceae) is used in Sudanese folk medicine for the treatment of jaundice and the fruits are used in Egypt for the treatment of diabetes.84 Apart coumarins, flavonoids, saponins and steroids which, have been isolated from B. aegyptiaca, new constituents such as N-trans-feruloyltyramine (131) and N-cis-feruloyltyramine (132) were isolated from the plant along with vanillic acid, syringic acid, and 3-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-1-propanone.92 These compounds were not tested for any biological activities. The work of de Abreu et al. led to the isolation and characterization of a new glycosylated lignan, 5-hydroxysesamin 5-O-β-D-glucopyranosyl-(1 → 2)-[β-D-glucopyranosyl-(1 → 6)]-β-D-glucopyranoside (133), from the leaves of Tabebuia argentea (Bignoniaceae) and a new phenolic glycoside, 1-benzyl-[6-p-hydroxybenzoyl]-β-D-glucopyranosyl-(1 → 3)-β-D-glucopyranoside, (134) from the petioles of Catalpa bignonioides (Bignoniaceae), along with five known phenolic glycosides; 1,6-di-O-p-hydroxybenzoyl-β-D-glucopyranoside, benzyl β-D-glucopyrano-side, 1-O-ethyl-6-(p-hydroxybenzoyl)-β-D-glucopyranoside, 1-O-p-hydroxybenzoyl-β-D-glucopyranoside, 6-p-hydroxybenzoyl-D-glucose (α,β), along six flavonol glycosides; kaempferol 3-O-β-D-glucopyranoside, quercetin 3-O-β-D-glucopyranoside, kaempferol 3-O-rutinoside, quercetin 3-O-sambubioside, quercetin 3-O-robinobioside, and rutin.93 T. tabebuia is used in Egyptian folk medicine as an anti-inflammatory and against influenza and C. bignonioides is known to have a sedative and a narcotic effect and it has also been used in the treatment of whooping cough and asthma. Many iridoids from these plants have been evaluated for their Hsp90 inhibitory activity.94 Jacaranda mimosaefolia (Bignoniaceae) is a useful medicinal plant whose bark decoction has been used to treat veneral diseases and to purify blood in Ecuador.95,96 The plant has also been reported to be used as an antisyphilitic, astringent and in applications against buboes.97 The phytochemistry of the stem bark of J. mimosaefolia afforded two new glycosides, 2-(3′,4′-dihydroxyphenyl) ethyl-3-O-α-L-rhamnopyranosyl-4-O-p-hydroxyphenylacetyl-6-O-caffeoyl-β-D-glucopyranoside (136) and 2-(3,4-dihydroxyphenyl) ethyl-3-O-α-L-rhamnopyranosyl-4-O-piperidine-3-carboxylic acid-6-O-caffeoyl-β-D-glucopyranoside (137). In addition, the known compounds lupeol (138), betulinaldehyde (139), terminic acid (140), betulinic acid (141), maslinic acid (142), β-sitosterol glucoside (143) and isoacteoside (135) were isolated and identified. Although different biological activities have been reported for this plant, such as antimicrobial activity98 and antioxidant activity,99 these new metabolites were not tested for any biological activities.
Bombax malabaricum DC (Bombacaceae: bignonias family) has been used in traditional medicine for the treatment of enteritis, dysentery, lymphadenoma, menorrhagia, and hepatitis.100,101 It has also been employed as a diuretic, demulcent, aphrodisiac, and an emetic and for curing impotence, in addition to showing significant anti-Heliobacter pylori activity.102 Seven flavones were isolated from the methanol extract of the flowers of B. malabaricum DC (Bombacaceae) and fourteen compounds from the n-hexane extract including cholesterol, stigmasterol, campestrol, α-amyrin and ten hydrocarbons. The seven flavonols include, vicenin 2 (144), linarin (145), saponarin (146), cosmetin (147), isovitexin (148), xanthomicrol (149) and apigenin (47)100 (Fig. 8). These pure compounds were not screened for biological activity but the n-hexane and methanol extracts showed significant antioxidant and antimicrobial activity.100 The n-hexane extract scavenged the free radical 1,1-diphenyl-2-picrylhydrazoyl (DPPH) over concentrations ranging between 0.55 and 0.0343 mg mL−1 and the methanol extract scavenged DPPH over the range 0.5–0.0312 mg mL−1. The maximum scavenging being observed was 0.55–0.5 mg mL−1 for both extracts. Both extracts were screened for antimicrobial activities against different bacterial, fungal and yeast strains using tetracycline (antibacterial) and fluconazole (antifungal) as standard drugs. The methanol extract showed significant activity against Stapylococcus aureus, Bacillus subtilis, Strptococcus faecalis (gram-positive strains), Escherichia coli, Neisserria gonorrhea, Pseudomonas aeruginosa (gram negative strains) and Candida albicans (yeast), but the hexane extract displayed between weak and moderate activity against the tested microorganisms. The n-hexane extract indicated no antifungal activity against Aspergillus niger and Aspergillus flavus (filamentous fungi) but the methanol extract revealed moderate activities.
Commiphora molmol, from the Burseraceae (torchwood family) is indigenous to desert areas of Somalia, Ethiopia and part of Kenya and exudes a gum (oleo-resin), which has been used as an insect repellent, antiseptic and an anti-inflammatory for the remedy of mouth and throat infections. In search of anti-staphylococcal agents from plants, Rahman et al. isolated two octanordammaranes; mansumbinone (150) and 3,4-seco-mansumbinoic acid (151) and two sesquiterpenes; β-elemene (152) and T-cadinol (153) (Fig. 8).103 These compounds were assessed for anti-staphylococcal activity against multi-drug and methicillin-resistant strains of Staphylococcus aureus. The most significant anti-staphylococcal activity was displayed by compound 151. It revealed the highest potency against the multi-drug effluxing strain SA1199B (MIC = 4 μg mL−1) two times more potent than the control antibiotic norfloxacin. The crude chloroform extract of the oleo-resin of C. molmol indicated potentiation of ciprofloxacin and tetracycline in several strains of the bacteria.103
5 Celastraceae, Chenopodiaceae, Cleomaceae, Cucurbitaceae and Cupressaceae
A summary of the medicinal uses and biological activities of the compounds of the plant families are indicated in Table 4, while chemical structures of identified metabolites are shown in Fig. 8 and 9.| Plant family | Plant name (Country) | Use in traditional medicine | Part of plant studied | Active principle | Measured activity | Author and Reference |
|---|---|---|---|---|---|---|
| Cleomaceae | Cleome paradoxa (Saudi Arabia) | Treatment of diabetes | Whole plant | 155, 156a, 156b | Not tested | Azza R. Abdel-Monem104 |
| Celastraceae | Maytenus senegalensis (Sudan) | Treatment of malaria | Root bark | 157 | Antiplasmodial, antileishmanial, cytotoxicity | Khalid et al.106 |
| Cucurbitaceae | Citrullus lanatus var. citroides (Sudan) | Treatment of rheumatism, swelling, gout; used a laxative | Fruit pulp | 158 | Anti-inflammatory | Abdelwahab et al.107 |
| Citrullus colcoynthis (Tunisia) | Treatment of rheumatism, hypertension and contagious infections. | Roots, stems and leaves | Extracts | Antibacterial, anticandidal | Marzouk et al.108 | |
| Cucurbita pepo (Egypt) | Treatment of diabetes, hypertension, tumor, immunomodulation; used as an antibacterial, antihypercholesterolemia, intestinal antiparasitia, anti-inflammatory | Ripe fruit | Extracts | Antimicrobia, cytotoxicity | Badr et al.109 | |
| Chenopodiaceae | Salsola tetranda (Tunisia) | Members of this genus are used in folk medicine as antihypertensive and for the treatment of tapeworm infestations. | Aerial parts | 165–172 | Antibacterial | Oueslati et al.110 |
| Salsola tetranda (Tunisia) | Treatment of hypertension, tapeworm infestations | Roots | 173, 174 | Antioxidant | Beyaoui et al.111 | |
| Salsola imbricate (Egypt) | Treatment of inflammations; used as a diuretic, an antioxidant and CNS depressant | Roots | 175, 176 | Not tested | Hamed et al.112 | |
| Cupressaceae | Juniperus phoenicea (Tunisia) | Treatment of cough; also used as a hypoglycaemic, an antiseptic and a diuretic | Berries (seeds) | Extracts | Antioxidant | Nasri et al.113 |
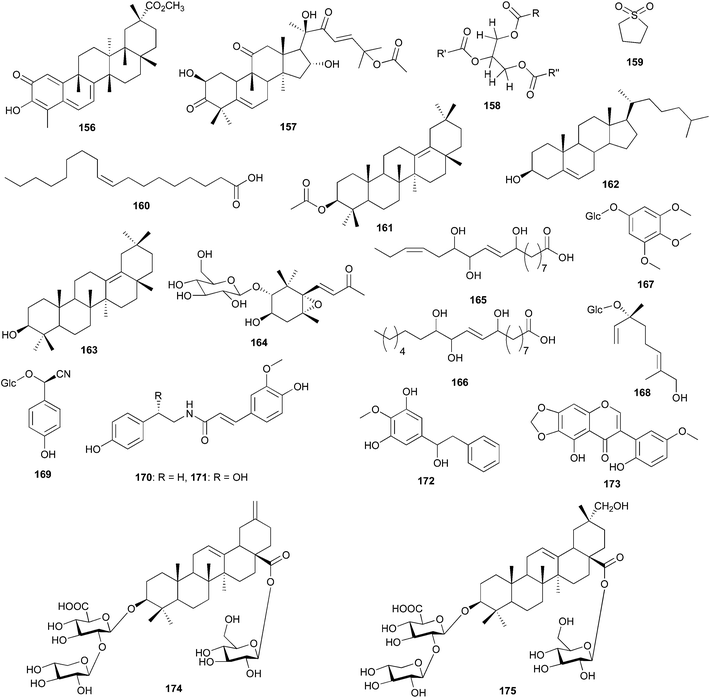
Cleome paradoxa (Cleomaceae) has shown noticeable antidiabetic activity.104,105 A new cembranoid diterpene, paradoxenoic acid (154) and a new alkaloid, paradoxonine (155a) and its tautomer (155b) were isolated and characterized from C. paradoxa. No biological activity has been reported of these metabolites. A decoction of the root bark of Maytenus senegalensis (Celastraceae: staff vine or bittersweet family) is widely used folk medicine in Sudan to treat malaria. Bioactivity-guided fraction of the root bark of this plant led to the isolation of a new quinonemethide triterpene, (20α)-3-hydroxy-2-oxo-24-nor-friedela-1(10),3,5,7-tetraen-carboxylic acid-(29)-methylester commonly known as pristimerin (156). Pristimerin revealed in vitro antiplasmodial activity against the chlorquine-resistant strain (Dd2) of Plasmodium falciparum (IC50 = 0.50 μg mL−1),106 thus supporting the local use of this plant as medication for malaria. Pristimerin also showed antileishmanial activity (IC50 = 6.8 ± 0.8 μg mL−1 against promostigotes of the WHO reference vaccine strain of Leishmania major. The cytotoxic activity of this compound was measured by the lymphocyte proliferation model and was detected at IC50 = 6.8 ± 0.8 μg mL−1.
Our survey of the Cucurbitaceae (gourd family) indicated three species (Table 4). These are Citrullus colocynthis, used in treating several diseases such rheumatism and hypertension, and many contagious diseases such as dermatological, gynecological, and pulmonary infections;107 Citrullus lanatus var. citroides used to treat rheumatism, swellings, gout and as a laxative108 and Cucurbita pepo L., used in the remedy of several ailments (antidiabetic, antihypertensive, antitumor, immunomodulation, antibacterial, antihypercholesterolemia, intestinal antiparasitia, antalgic).109 The antibacterial and anticandidal activities of the aqueous and diluted acetone extracts of various parts (roots, stems, leaves, fruits and seeds) of C. colocynthis were measured against gram-positive and gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Enterococcus faecalis) and various Candida spp. (Candida glabrata, Candida albicans, Candida parapsilosis and Candida kreusei).107 All the extracts showed activity against all the strains with the highest activity from the fruit aqueous extracts (MIC 0.10 mg mL−1 against Candida albicans and Candida glabrata, 0.20 mg mL−1 against Escherichia coli and Pseudomonas aeruginosa) and the lowest activity from the roots. These studies validate the use of this plant as a broad-spectrum antimicrobial agent. Cucurbitacin E (157), extracted from the powdered fruit pulp of Citrullus lanatus exhibited in vitro and in vivo anti-inflammatory activity through the inhibition of COX and RNS enzymes but not reactive oxygen species (ROS), corroborating the use of this plant as anti-inflammatory. In addition, compound 157 does not affect normal human liver cells. Badr et al. separated six compounds from the rind, flesh and seeds of the pumpkin, Cucurbita pepo.109 These compounds include, a triglyceride fatty acid mixture (158), tetrahydrothiophene (159), linoleic acid (160), calotropoleanly ester (161), cholesterol (162), and 13(18)-oleanen-3-ol (163). Extracts of the various parts of this plant were screened for antimicrobial, antiviral, antitumor and cytotoxicity activities in comparison with the isolated components.109 The rind and flesh extracts revealed moderate antimicrobial activity against gram-positive bacteria, Bacillus subtilis and Bacillus cereus, while the oil from the seeds displayed strong antifungal activity against Saccharomyces cerevesiae. On the contrary, the defatted seed showed no antimicrobial activity. Extracts of the rind, flesh and the defatted seed showed significant antiviral activity against Live Newcastle Disease Virus (NDV) vaccine strains and Live Infectious Bursitis Viruses (IBDV) vaccine strain, D78 in the range 4–6 μL mL−1 at therapeutic indices of >100, >80 and 50 respectively. Extracts of different parts of the pumpkin (rind, flesh, seed oil and defatted seeds) showed potent in vitro antitumor activities against liver carcinoma (Hepg2) (IC50 range from 0.6–5.03 μg). Apart from the seed oil, the extracts exhibited cytotoxicity activity against breast carcinoma (MCF7), with an IC50 in the range 0.40–1.01 μg. Comparing the extracts with the pure compounds, only linoleic acid (160) showed potent antimicrobial activity against gram-positive bacteria (Streptomyces viridochromogenes) and moderate activity against yeast (Candida albicans) and the fungi, Mucor miehi. The other compounds were inactive. Linoleic acid exhibited potent cytotoxic activity against brine shrimp compared to the rind and flesh extracts, while the other isolated compounds were inactive against brine shrimp. The use of the plant as a remedy for several diseases is justified.
The genus Salsola (Chenopodiaceae: goosefoot family) consists of several species found mostly in dry parts of Africa, Asia and Europe, very few of them having been utilized in folk medicine. Oueslati et al. have isolated the new norisoprenoid 3β-hydroxy-5α,6α-epoxy-β-ionone-2α-O-β-D-glucopyranoside (164) and the long-chain hydroxyl fatty acids 9,12,13-trihydroxyoctadeca-10(E),15(Z)-dienoic acid (165) and 9,12,13-trihydroxyoctadeca-10(E)-dienoic acid (166) from Salsola tetrandra aerial parts, together with 3,4,5-trimethoxyphenyl-β-D-glucopyranoside (167), 9-hydroxylinaloyl glucoside (168), taxiphyllin (169), trans-N-feruloyltyramine (170), and S-(−)-trans-N-feruloyloctopamine (171).110 All the eight compounds were screened for antimicrobial activity against gram-positive evaluated against Gram-positive Staphylococcus aureus ATCC 29213, Staphylococcus epidermidis NCIMB 8853, and Micrococcus luteus NCIMB 8166 and gram-negative Escherichia coli ATCC 35218 and Pseudomonas aeruginosa ATCC 27853 using microdilution methods on liquid medium. Compounds 169 and 171 revealed moderate antimicrobial activity by preventing the growth of S. aureus (MIC 200 μg mL−1). Two new compounds, tetranins A and B, 1-(3,5-dihydroxy-4′-methoxyphenyl)-2 phenylethanol (172) and 5,2′-dihydroxy-5′-methoxy-6,7-methylenedioxy-isoflavone (173), were isolated from the EtOAc extract of Salsola tetrandra roots.111 The two compounds displayed significant antioxidant effect in the DPPH and 2,2′-azinobis(3-ethylbenzothiazoline)-6-sulphonic acid (ABTS) assays. Another Salsola species, widely growing in Egypt, which has been studied, is S. imbricata from which two new triterpene glycosides have been isolated and characterized but not biologically screened: 3-O-β-D-xylopyranosyl-(1 → 2)-O-β-D-glucuronopyranosyl-akebonic acid 28-O-β-D-glucopyranoside (174) and 3-O-β-D-xylopyranosyl-(1 → 2)-O-β-D-glucuronopyranosyl-29-hydroxyoleanolic acid 28-O-β-D-glucopyranoside (175).112 The extracts of the berries of Juniperus phoenicea (Cupressaceae: cypress family) indicated significant antioxidant activity in the DPPH and ABTS assay.113 The berries of the plant are rich in minerals, unsaturated lipids and polyphenols which may account for their antioxidant properties.
6 Conclusions
The results presented in this review represent an overview of the biological activities of selected NPs isolated from plants used in traditional medicine in North Africa, which could be useful in drug discovery programs. Our intention has been to focus on those plants whose ethnobotanical uses correlate with the biological activities of the derived NPs. Even though this report does not claim to be exhaustive, the goal of documenting the baseline knowledge, from which further investigations could be carried out, has been achieved. The plant sources, geographical collection sites, chemical structures of pure compounds as well as their spectroscopic data, were retrieved from literature sources comprising data collected from major international journals on natural products and some available PhD theses, spanning the period 1971 to 2014. Our survey consisted in collecting data from the literature sources, mainly using author queries in major natural product and medicinal chemistry journals. The collected data includes plant sources, uses of plant material in traditional medicine, plant families, region of collection of plant material, isolated metabolites and type (e.g. flavonoid, terpenoid, etc.), measured biological activities, etc. The data was collected on a Excel sheet and analyzed. In the second part of this review series, emphasis will be on the remaining plant families.Acknowledgements
Financial support is acknowledged from Lhasa Ltd, Leeds, UK through the Chemical and Bioactivity Information Centre (CBIC), University of Buea, Cameroon. Dr Philip N. Judson (CBIC, UK) is acknowledged for proof reading the original manuscript prior to submission.Notes and references
- N. S. Abdel-Azim, K. A. Shams, A. A. A. Shahat, M. M. El Missiry, S. I. Ismail and F. M. Hammouda, Res. J. Med. Plant, 2011, 5, 136 CrossRef.
- A. A. Shahat, L. Pieters, S. Apers, N. M. Nazeif, N. S. Abdel-Azim, D. V. Berghe and A. J. Vlietinck, Phytother. Res., 2001, 15, 593 CrossRef CAS PubMed.
- L. Dagmar, International trade inmedicinal and aromatic plants, actors, volumes and commodities, plants, in Medicinal and aromatic plants, ed. R. J. Bogers, L. E. Craker and D. Lange, Berlin, Heidelberg, Springer, 2006 Search PubMed.
- United Nations Organization, Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings, http://millenniumindicators.un.org/unsd/methods/m49/m49regin.htm, assessed on 8th July 2014 Search PubMed.
- M. Vilà, Y. Meggaro and E. Weber, Orsis, 1999, 14, 9 Search PubMed.
- TDWG, The World Geographical Scheme for Recording Plant Distributions-The STANDARDS-2nd edition, 2001, http://www.tdwg.org/standards/109, accessed on 8th July 2014 Search PubMed.
- F. Ntie-Kang, J. A. Mbah, L. M. Mbaze, L. L. Lifongo, M. Scharfe, J. Ngo Hanna, F. Cho-Ngwa, P. A. Onguéné, L. C. O. Owono, E. Megnassan, W. Sippl and S. M. N. Efange, BMC Complementary Altern. Med., 2013, 13, 88 CrossRef PubMed.
- F. Ntie-Kang, P. A. Onguéné, M. Scharfe, L. C. O. Owono, E. Megnassan, L. M. Mbaze, W. Sippl and S. M. N. Efange, RSC Adv., 2014, 4, 409 RSC.
- F. Ntie-Kang, P. A. Onguéné, G. W. Fotso, K. Andrae-Marobela, M. Bezabih, J. C. Ndom, B. T. Ngadjui, A. O. Ogundaini, B. M. Abegaz and L. M. Mbaze, PLoS One, 2014, 9(3), e90655 Search PubMed.
- F. Ntie-Kang, D. Zofou, S. B. Babiaka, R. Meudom, M. Scharfe, L. L. Lifongo, J. A. Mbah, L. M. Mbaze, W. Sippl and S. M. N. Efange, PLoS One, 2013, 8(10), e78085 CAS.
- F. Ntie-Kang, L. L. Lifongo, J. A. Mbah, L. C. O. Owono, E. Megnassan, L. M. Mbaze, P. N. Judson, W. Sippl and S. M. N. Efange, In Silico Pharmacol., 2013, 1, 12 CrossRef.
- F. Ntie-Kang, J. A. Mbah, L. L. Lifongo, L. C. O. Owono, E. Megnassan, L. M. Mbaze, P. N. Judson, W. Sippl and S. M. N. Efange, Org. Med. Chem. Lett., 2013, 3, 10 CrossRef PubMed.
- P. A. Onguéné, F. Ntie-Kang, J. A. Mbah, L. L. Lifongo, J. C. Ndom, W. Sippl and L. M. Mbaze, Org. Med. Chem. Lett., 2014, 4, 6 CrossRef PubMed.
- F. Ntie-Kang, L. L. Lifongo, L. M. Mbaze, N. Ekwelle, L. C. O. Owono, E. Megnassan, P. N. Judson, W. Sippl and S. M. N. Efange, BMC Complementary Altern. Med., 2013, 13, 147 CrossRef PubMed.
- D. Zofou, F. Ntie-Kang, W. Sippl and S. M. N. Efange, Nat. Prod. Rep., 2013, 30, 1098 RSC.
- L. L. Lifongo, C. V. Simoben, F. Ntie-Kang, S. B. Babiaka and P. N. Judson, Nat. Prod. Bioprospect., 2014, 4, 1 CrossRef PubMed.
- F. Ntie-Kang, L. L. Lifongo, C. V. Simoben, S. B. Babiaka, W. Sippl and L. M. Mbaze, RSC Adv., 2014, 4, 28728 RSC.
- F. Ntie-Kang, L. L. Lifongo, C. V. Simoben, S. B. Babiaka, W. Sippl and L. M. Mbaze, RSC Adv., 2014, 4, 35348 RSC.
- C. V. Simoben, F. Ntie-Kang, L. L. Lifongo, S. B. Babiaka, W. Sippl and L. M. Mbaze, RSC Adv., 2014, 4, 40095 RSC.
- P. A. Onguéné, F. Ntie-Kang, L. L. Lifongo, J. C. Ndom, W. Sippl and L. M. Mbaze, Malar. J., 2013, 13, 449 CrossRef PubMed.
- F. Ntie-Kang, P. A. Onguéné, L. L. Lifongo, J. C. Ndom, W. Sippl and L. M. Mbaze, Malar. J., 2014, 13, 81 CrossRef PubMed.
- J. N. Yong and F. Ntie-Kang, Anti-Infect. Agents, 2014, 12, 178 CrossRef CAS.
- J. N. Nwodo, A. Ibezim, C. V. Simoben and F. Ntie-Kang, Curr. Med. Chem.: Anti-Cancer Agents, 2014 Search PubMed , in press.
- S. Miladi and M. Damak, J. Soc. Chim. Tunis., 2008, 10, 101 CAS.
- G. O. M. ELhassan, A. Adhikari, S. Yousuf, M. H. Rahman, A. Khalid, H. Omer, H. K. Fun, H. Jahan, M. I. Choudhary and S. Yagi, Phytochem. Lett., 2012, 5, 725 CrossRef CAS PubMed.
- U. Eggli, Illustrated handbook of succulent plants: Monocotyledons, Springer, 2001 Search PubMed.
- D. Grindly and T. Reynolds, J. Ethnopharmacol., 1986, 16, 117 CrossRef.
- MAPRI, Review of Trade in Wildlife Medicinal Plant in Khartoum. Medicinal and Aromatic Plant Research Institute, Khartoum, Sudan, 1997, p. 9 Search PubMed.
- H. Khalid, W. E. Abdalla, H. Abdelgadir, T. Opatz and T. Efferth, Nat. Prod. Bioprospect., 2012, 2, 92 CrossRef CAS PubMed.
- E. Abdel-Sattar, A. A. Zaitoun, M. A. Farag, S. H. El Gayed and F. M. H. Harraz, Nat. Prod. Res., 2010, 24, 226 CrossRef CAS PubMed.
- J. A. Duke, Handbook of medicinal herbs, CRC Press, 1985, Boca Raton, p. 843 Search PubMed.
- A. A. Ferrero, C. Sanchez Chopa, J. O. Werdin Gonzalez and R. A. Alzogaray, Fitoterapia, 2007, 78, 311 CrossRef CAS PubMed.
- A. A. Ferrero, J. O. Werdin Gonzalez and C. Sanchez Chopa, Fitoterapia, 2006, 77, 381 CrossRef CAS PubMed.
- S. B. Glisic, D. R. Misic, M. D. Stamenic, I. T. Zizovic, R. M. Asanin and D. U. Skala, Food Chem., 2007, 105, 346 CrossRef CAS PubMed.
- A. A. Ahmed, M. M. Bishr, M. A. El-Shanawany, E. Z. Attia, S. A. Ross and P. W. Pare, Phytochemistry, 2005, 66, 1680 CrossRef CAS PubMed.
- Y. Kumarasamy, L. Nahar, M. Byres, A. Delazar and S. D. Sarker, J. Herb. Pharmacother., 2005, 5, 61 CAS.
- H. Marzouki, A. Khaldi, D. Falconieri, A. Piras, B. Marongiu, P. Molicotti and S. Zanetti, Nat. Prod. Commun., 2010, 5, 1955 CAS.
- H. Ozbek, S. Ugras, H. Dulger, I. Bayram, I. Tuncer, G. Ozturk and A. Ozturk, Fitoterapia, 2003, 74, 317 CrossRef CAS.
- S. M. Ezzat, H. M. Abdallah, G. A. Fawzy and S. A. El-Maraghy, Nat. Prod. Res., 2012, 26, 282 CrossRef CAS PubMed.
- D. L. Lucetti, E. C. P. Lucetti, M. A. M. Bandeira, H. N. H. Veras, A. H. Silva, L. K. A. M. Leal, A. A. Lopes, V. C. C. Alves, G. S. Silva, G. A. Brito and G. B. Viana, J. Inflammation, 2010, 7, 60 CrossRef CAS PubMed.
- G. Kweifio-Okai and A. R. Carroll, Phytother. Res., 1993, 7, 213 CrossRef CAS.
- M. F. Otuki, F. Vieira-Lima, A. Malheiros, R. A. Yunes and J. B. Calixto, Eur. J. Pharmacol., 2005, 507, 253 CrossRef CAS PubMed.
- R. Medeiros, M. F. Otuki, M. C. W. Avellar and J. B. Calixto, Eur. J. Pharmacol., 2007, 559, 227 CrossRef CAS PubMed.
- T. Narender, T. Khaliq, A. B. Singh, M. D. Joshi, P. Mishra, J. P. Chaturvedi, A. K. Srivastava, R. Mauryaa and S. C. Agarwal, Eur. J. Med. Chem., 2009, 44, 1215 CrossRef CAS PubMed.
- B. Singh and S. Singh, Phytother. Res., 2003, 17, 814 CrossRef CAS PubMed.
- M. F. Otuki, J. Ferreira, F. V. Lima, C. Meyre-Silva, A. Malheiros, L. A. Muller, G. S. Cani, A. R. Santos, R. A. Yunes and J. B. Calixto, J. Pharmacol. Exp. Ther., 2005, 313, 310 CrossRef CAS PubMed.
- Y. S. Ravikumar, K. M. Mahadevan, H. Manjunatha and N. D. Satyanarayana, Phytomedicine, 2010, 17, 513 CrossRef CAS PubMed.
- G. S. Jeong, B. Li, D. S. Lee, K. H. Kim, I. K. Lee, K. R. Lee and Y. C. Kim, Int. Immunopharmacol., 2010, 10, 1587 CrossRef CAS PubMed.
- R. P. Singh, P. Agrawal, D. Yim, C. Agarwal and R. Agarwal, Carcinogenesis, 2005, 26, 845 CrossRef CAS PubMed.
- Y. L. Hsu, P. L. Kuo and C. C. Lin, Biochem. Pharmacol., 2004, 67, 823 CrossRef CAS PubMed.
- M. H. Pan, C. S. Lai, Y. J. Wang and C. T. Ho, Biochem. Pharmacol., 2006, 72, 1293 CrossRef CAS PubMed.
- Y. L. Hsu, P. L. Kuo, C. F. Liu and C. C. Lin, Cancer Lett., 2004, 212, 53 CrossRef CAS PubMed.
- H. Y. Shim, J. H. Park, H. D. Paik, S. Y. Nah, D. S. H. L. Kim and Y. S. Han, Mol. Cells, 2007, 24, 95 CAS.
- J. M. Rollinger, A. Hornick, T. Langer, H. Stuppner and H. Prast, J. Med. Chem., 2004, 47, 6248 CrossRef CAS PubMed.
- S. Y. Kang, S. H. Sung, J. H. Park and Y. C. Kim, Arch. Pharmacal Res., 1998, 21, 718 CrossRef CAS.
- Z. Ding, Y. Dai and Z. Wang, Planta Med., 2005, 71(2), 183 CrossRef CAS PubMed.
- T. Valle, J. L. López, J. M. Hernández and P. Corchete, Plant Sci., 1997, 125, 97 CrossRef CAS.
- T. Ojala, S. Remes, P. Haansuu, H. Vuorela, R. Hiltunen, K. Haahtela and P. Vuorel, J. Ethnopharmacol., 2000, 73, 299 CrossRef CAS.
- N. Nenadis, H. Y. Zhang and M. Z. Tsimidou, J. Agric. Food Chem., 2003, 51, 1874 CrossRef CAS PubMed.
- H. Kikuzaki, M. Hisamoto, K. Hirose, K. Akiyama and H. Taniguchi, J. Agric. Food Chem., 2002, 50, 2161 CrossRef CAS PubMed.
- M. Ohnishi, T. Matuo, T. Tsuno, A. Hosoda, E. Nomura, H. Taniguchi, H. Sasaki and H. Morishita, Biofactors, 2004, 21, 315 CrossRef CAS.
- B. L. Garcia, A. S. Ball, J. Rodriguez, M. I. Pérez-Leblica, M. E. Ariasa and J. L. Copa-Patiño, FEMS Microbiol. Lett., 1998, 158, 95 CrossRef CAS PubMed.
- M. Jin, J. H. Yang, E. Lee, Y. Lu, S. Kwon, K. H. Son, J. K. Son and H. W. Chang, Biol. Pharm. Bull., 2009, 32, 1500 CAS.
- C. Hu and D. D. Kitts, Mol. Cell. Biochem., 2004, 265, 107 CrossRef CAS.
- R. N. Yadava and M. Raj, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2012, 51, 635 Search PubMed.
- L. S. Ooi, H. Wang, Z. He and V. E. Ooi, J. Ethnopharmacol., 2006, 106, 187 CrossRef CAS PubMed.
- K. K. Chiruvella, A. Mohammed, G. Dampuri, R. G. Ghanta and S. C. Raghavan, Int. J. Biomed. Sci., 2007, 3, 269 CAS.
- M. Dahia, L. Siracusa, H. Laouer and G. Ruberto, Nat. Prod. Commun., 2009, 4, 1691 CAS.
- Y. Miyamae, M. Kurisu, J. Han, H. Isoda and H. Shigemori, Chem. Pharm. Bull., 2011, 59, 502 CrossRef CAS.
- M. E. S. Kassem, S. Shoela, M. M. Marzouk and A. A. Sleem, Nat. Prod. Res., 2012, 26(15), 1381 CrossRef CAS PubMed.
- K. H. Shaker, N. Morsy, H. Zinecker, J. F. Imhoff and B. Schneider, Phytochem. Lett., 2010, 3, 212 CrossRef CAS PubMed.
- V. L. Kumar and S. Arya, Medicinal uses and pharmacological properties of Calotropis procera, in Recent Progress in Medicinal Plants 11, ed. J. N. Govil, Studium Press, Houston, TX, USA, 2006, pp. 373–388 Search PubMed.
- T. Choedon, G. Mathan, S. Arya, V. L. Kumar and V. Kumar, World J. Gastroenterol., 2006, 12, 2517 Search PubMed.
- A. Basu, T. Sen, R. N. Ray and A. K. Nag Chaudhuri, Fitoterapia, 1992, 63, 507 Search PubMed.
- S. R. M. Ibrahim, G. A. Mohamed, L. A. Shaala, L. M. Y. Banuls, G. V. Goietsenoven, R. Kiss and D. T. A. Youssef, Phytochem. Lett., 2012, 5, 490 CrossRef CAS PubMed.
- Z. Y. Babaamer, L. Sakhri, H. I. Al-Jaber, M. A. Al-Qudah and M. H. Abu Zarga, J. Asian Nat. Prod. Res., 2012, 14, 1137 CrossRef CAS PubMed.
- M. Laid, Etude Phytochimique d'une Plante Medicinale de l'Est Algerien (Artemisia herba alba), Thèse de Doctorat des Sciences en Chimie Organique, Faculté des Sciences Exactes, Universite Mentouri Constantine, 2011.
- M. E. Hegazy, H. Matsuda, S. Nakamura, T. A. Hussein, M. Yoshikawa and P. W. Paré, Phytochemistry, 2014, 103, 154 CrossRef CAS PubMed.
- T. A. Al-Howiriny, A. J. Al-Rehaily, J. R. Pols, J. R. Porter, J. S. Mossa and B. Ahmed, Nat. Prod. Res., 2005, 19, 253 CrossRef CAS PubMed.
- M. A. Hussein, Free Radicals Antioxid., 2011, 1, 49 CrossRef CAS.
- J. Duke, Handbook of phytochemical constituents of gras herbs and other economic plants, Boca, Raton, FL, CRC Press, 1992 Search PubMed.
- X. L. Shen, M. Nielsen, M. R. Witt, O. Sterner, O. Bergendorff and M. Khayyal, Zhongguo Yaoli Xuebao, 1994, 15(5), 385 CAS.
- N. Boutaghane, L. Voutquenne-Nazabadioko, A. Simon, D. Harakat, K. Benlabed and Z. Kabouche, Phytochem Lett., 2013, 6, 519 CrossRef CAS PubMed.
- M. K. Boukef, Les plantes dans la médecine traditionnelle tunisienne, Collection: agence de cooperation culturelle et technique, 1986 Search PubMed.
- J. Bellakhadar, La pharmacopée marocaine traditionnelle, Ibis Press, Paris, 1997 Search PubMed.
- J. Dai, C. Zhao, Q. Zhang, Z. L. Liu, R. Zheng and L. Yang, Phytochemistry, 2001, 58, 1107 CrossRef CAS.
- M. M. Salama, Z. A. Kandil and W. T. Islam, Nat. Prod. Res., 2012, 26(22), 2057 CAS.
- M. A. Ahmed, A. El-Mawlaa, S. F. Faraga and T. Beuerleb, Nat. Prod. Res., 2011, 25(1), 45 CrossRef PubMed.
- S. Mabrouk, A. Elaissi, H. B. Jannet and F. Harzallah-Skhiri, Nat. Prod. Res., 2011, 25(1), 77 CrossRef CAS PubMed.
- L. Hammoud, R. Seghiria, S. Benayache, P. Mosset, A. Lobstein, M. Chaabi, F. León, I. Brouard, J. Bermejo and F. Benayache, Nat. Prod. Res., 2012, 26(3), 203 CrossRef CAS PubMed.
- M. M. Abou-Zaid, Z. El-Karemy, S. I. El-Negoumy, I. Altosaar and N. A. M. Saleh, Bull. Chem. Soc. Ethiop., 1991, 5(1), 37 CAS.
- S. D. Sarker, B. Bartholomew and R. J. Nash, Fitoterapia, 2000, 71, 328 CrossRef CAS.
- M. B. de Abreu, A. Temraz, A. Vassallo, A. Braca and N. de Tommasi, Phytochem. Lett., 2014, 7, 85 CrossRef PubMed.
- F. Dal Piaz, A. Vassallo, A. Temraz, R. Cotugno, M. A. Belisario, G. Bifulco, M. G. Chini, C. Pisano, N. de Tommasi and A. Braca, J. Med. Chem., 2013, 56, 1583 CrossRef PubMed.
- A. M. Zaghloul, A. A. Gohara, M. M. Ahmad, H. N. Baraka and A. A. El-Bassuony, Nat. Prod. Res., 2011, 25(1), 68 CrossRef CAS PubMed.
- M. S. Gachet and W. Schühly, J. Ethnopharmacol., 2009, 121, 14 CrossRef CAS PubMed.
- J. M. Watt and M. G. Breyer-Brandwijk, The medicinal and poisonous plants of Southern and Eastern Africa, E. & S. Livingstone Ltd, Edinburgh and London, 1962, 2nd edn, pp. 142–144 Search PubMed.
- J. J. Rojas, V. J. Ochoa, S. A. Ocampo and J. F. Muñoz, BMC Complementary Altern. Med., 2006, 6, 2 CrossRef PubMed.
- W. Choi, S. Lee, H. Lee, Y. Lee and B. Park, Han'guk Nonghwa Hakhoechi, 1998, 41, 556 CAS.
- A. M. El-Hagrassi, M. M. Ali, A. F. Osman and M. Shaaban, Nat. Prod. Res., 2011, 25(2), 141 CrossRef CAS PubMed.
- T. Yang, K. Chen, C. Ch'en and Y. Kao, Taiwan Kexue, 1970, 24, 15 CAS.
- Y. Wang and T. Huang, FEMS Immunol. Med. Microbiol., 2005, 43, 295 CrossRef CAS PubMed.
- M. M. Rahman, M. Garvey, L. J. V. Piddock and S. Gibbons, Phytother. Res., 2008, 22, 1356 CrossRef CAS PubMed.
- A. R. Abdel-Monem, Nat. Prod. Res., 2012, 26(3), 264 CrossRef CAS PubMed.
- E. Abdel-Sattar, A. R. Abdel-Monem and A. A. Sleem, B.Br. Pharmacognosy Res., 2009, 1, 175 CAS.
- S. A. Khalid, G. M. Friedrichsen, S. B. Christensen, A. El Tahir and G. M. Satti, ARKIVOC, 2007, ix, 129 CrossRef.
- B. Marzouk, Z. Marzouk, R. Décor, H. Edziri, E. Haloui, N. Fenina and M. Aouni, J. Ethnopharmacol., 2009, 125, 344 CrossRef PubMed.
- S. I. Abdelwahab, L. E. A. Hassan, H. M. Sirat, S. M. A. Yagi, W. S. Koko, S. Mohan, M. M. E. Taha, S. Ahmad, C. S. Chuen, P. Narrima, M. M. Rais and A. H. A. Hadi, Fitoterapia, 2011, 82, 1190 CrossRef CAS PubMed.
- S. E. A. Badr, M. Shaaban, Y. M. Elkholy, M. H. Helal, A. S. Hamza, M. S. Masoud and M. M. El Safty, Nat. Prod. Res., 2011, 25(6), 1524 CrossRef CAS PubMed.
- M. H. Oueslati, H. B. Jannet, Z. Mighri, J. Chriaa and P. M. Abreu, J. Nat. Prod., 2006, 69, 1366 CrossRef CAS PubMed.
- A. Beyaoui, A. Chaari, H. Ghouila, M. A. Hamza and H. B. Jannet, Nat. Prod. Res., 2012, 26(3), 235 CrossRef CAS PubMed.
- A. I. Hamed, M. Masullo, M. G. Sheded, U. A. Mahalel, M. M. Tawfik, A. Perrone and S. Piacente, Phytochem. Lett., 2011, 4, 353 CAS.
- N. Nasri, N. Tlili, W. Elfalleh, E. Cherif, A. Ferchichi, A. Khaldi and S. Triki, Nat. Prod. Res., 2011, 25(18), 1733 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |