Palladium nanoparticles supported on natural nanozeolite clinoptilolite as a catalyst for ligand and copper-free C–C and C–O cross coupling reactions in aqueous medium†
Abstract
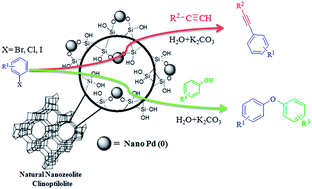
In this article, Pd0-nanoparticles supported on natural nanozeolite clinoptilolite (CP) were successfully synthesized, and the catalytic activity of the nanocatalyst was investigated in C–C and C–O coupling reactions, namely the Sonogashira and Ullmann condensation reactions in an aqueous medium. The nanocatalyst was characterized by various techniques such as powder-XRD, BET, SEM, TEM, HRTEM, TEM-EDS, ICP-OES and XPS. From an electron microscopy (SEM) study it can be inferred that the particles are mostly spherical or of ellipsoidal shape and have diameters ranging from 25 to 80 nm. A HRTEM study reveals that Pd0-nanoparticles supported on nanozeolite CP have an average diameter of ∼10 nm. The supported palladium nanoparticles showed excellent catalytic activity in the synthesis of aryl alkynes and diaryl ethers in high yields. In addition, the nanocatalyst could be recycled and reused several times without significant loss of catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...