Kinetics and mechanism of diallyl sulfoxide pyrolysis; a combined theoretical and experimental study in the gas phase†
Abstract
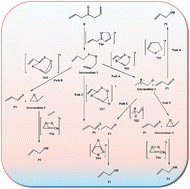
A combined experimental and computational study was carried out on the gas phase pyrolysis reaction of diallylsulfoxide. Allyl alcohol and thioacrolein were detected as the major products during a unimolecular reaction. Experimental kinetic studies were carried out via a static system under the pressure of 21–55 torr and temperature of 435.2–475.1 K. Based on the experiments, the reaction is homogeneous and proceeds through a zwitterionic intermediate. Computational studies at the DFT (B3LYP) and QCISD(T) levels with 6-311++G(d,p) basis set indicated a two-step concerted pathway as the possible route. Comparison between the experimental and theoretical activation parameters for the most probable path confirmed a good agreement.

 Please wait while we load your content...
Please wait while we load your content...