Screening of Coffea spp. honey by different methodologies: theobromine and caffeine as chemical markers
Abstract
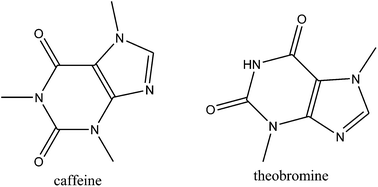
Coffea spp. honey was screened by UV/VIS, HS-SPME/GC-MS/FID, USE/GC-MS/FID and HPLC-DAD. The direct HPLC-DAD methodology overcame the major limitations of the other methods used. The obtained results constitute a breakthrough since dominant xanthine derivatives were found (theobromine and caffeine with HPLC-DAD and caffeine by USE/GC-MS/FID). Phenylacetaldehyde was the major headspace compound.

 Please wait while we load your content...
Please wait while we load your content...