Potential of grape seed-derived polyphenols extract for protection against testosterone-induced benign prostatic hyperplasia in castrated rats
Yongfang Leia,
Dong Liua,
Xiuhua Rena and
Jinglou Chen*b
aDepartment of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
bDepartment of Pharmacy, Puai Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. E-mail: jinglouchen@126.com; Fax: +86-027-68835014; Tel: +86-027-68835014
First published on 5th November 2014
Abstract
This study was to investigate the potential of grape seed-derived polyphenols extract (GSP) to protect against testosterone-induced benign prostatic hyperplasia (BPH) in castrated rats. After a 5 week experimental period, the prostatic levels of proinflammatory cytokines and plasma androgen level were measured by enzyme linked immunosorbent assay. Prostatic oxidative stress was evaluated by detecting the activities of antioxidant enzymes. Additionally, the prostatic levels of extracellular signal-regulated kinases (ERK), p38, protein kinase B (PKB/AKT), nuclear factor (NF)κB and intercellular cell adhesion molecule (ICAM) were determined using western blot analysis. It was found that GSP ameliorated the testosterone-induced high androgen level, over-expressions of NFκB and ICAM, and the high phosphorylation levels of ERK, p38 and AKT, as well as normalized antioxidant enzyme activities and regulated the proinflammatory cytokines. These results suggested that GSP had prostatic protective nature via regulating the androgen-MAPK/AKT-ICAM pathway and eventually alleviating the prostatic inflammatory responses and oxidative stress.
1. Introduction
Benign prostatic hyperplasia (BPH) is a major chronic disease in andriatry and is already one of the most common disorders in elderly men.1 Reports show that BPH is a kind of benign proliferation and is likely to cause lower urinary tract symptoms.2 Furthermore, patients with BPH tend to experience an increased risk of prostate cancer since both of them are related to the prostatic chronic inflammatory state, disordered hormone level and uncontrolled angiogenesis in vivo.3,4 Research for highlighting the progression of BPH has been ongoing for years. However, both the pathology and etiology of BPH still prove to be elusive.5 Additionally, it is reported that patients with prostatic problems are more susceptible to depressive disorders.6 So, the physical, mental and social well-being of patients are seriously obstructed.Surgery and drug therapies are the most common treatments for BPH. Obviously, drug therapies have much more advantages. Unfortunately, there are usually several adverse effects during the long term medication period.7 Finasteride tablet, a 5-α-reductase (5AR) inhibitor and set as the positive control in this study, is one of the most commonly used clinical agents for the treatment of BPH.2 However, good therapeutic effect seems to only emerge many years later from the beginning of the treatment.8
Patients with BPH are often characterized by chronic inflammatory state and oxidative damage. It is not only that the prostate growth is under the indirect control of androgens through mediating growth factors, but also that many cytokines with a wide range of biological activities are secreted in the prostatic microenvironment.9,10 Thus, herbal remedies with proven anti-inflammatory and antioxidant activities have been used in the treatment of prostatitis and BPH for years.11 Particularly, natural polyphenolic substances with excellent antioxidant activity have attracted much attention for their possible prostate protective nature.7,12 Grape (Vitis vinifera) is a member of the Vitaceae family and traditionally used in Chinese folk medicine for the treatment of promoting blood circulation, eliminating edema, relieving restlessness, diuretics and nourishing the kidney (painful urination, prostatitis and BPH) for patients suffering from kidney/prostate problems due to its satisfactory therapeutic effectiveness.13 The main components of grape seeds are natural polyphenolic substances (especially procyanidins). Studies demonstrate that grape seed-derived polyphenols extract (GSP) has many benefits, such as anti-inflammatory, improving lipid metabolism, limiting adipogenesis, anti-diabetic and cardioprotective.14 Especially, GSP is far more active on scavenging free radicals and inhibiting lipid peroxidation than the popular antioxidant vitamin C, which is suggested to be a potential anti-BPH agent.4,15 In addition, GSP is reported to have the ability to inhibit the growth of prostate cancer PC-3 cells with a possible mechanism of modulating the antioxidant–prooxidant balance.16
This study was undertaken to investigate the potential of GSP to protect against testosterone-induced BPH in castrated rats and explore the possible mechanisms.
2. Materials and methods
2.1. Reagents
Standardized GSP was prepared according to our previous study.17 The content of (+)-catechin, (−)-epicatechin and procyanidin B2 in GSP was 62.4 ± 1.3, 30.5 ± 0.8 and 11.5 ± 0.3 mg per g GSP, respectively. The total content of procyanidin B1-4 in GSP was 39.4 ± 0.8 mg per g GSP and the total content of polyphenol in GSP was 650 ± 36.0 mg per g GSP. Finasteride tablets were purchased from Merck Sharp & Dohme, Ltd. (Hoddesdon, Hertfordshire, UK). Testosterone propionate was obtained from Shanghai General Pharmaceutical Co Ltd. (Shanghai, China). The commercial kits for the analysis of the prostatic acid phosphatase (PACP), superoxide dismutase (SOD), malondialdehyde (MDA) and glutathione peroxidase (GPx) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). The bicinchoninic acid (BCA) kit used for the detection of protein content was purchased from Beyotime Institute of Biotechnology (Shanghai, China). The commercial enzyme linked immunosorbent assay (ELISA) kits used for the measurement of prostatic levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and cyclooxygenase-2 (COX-2), and plasma levels of dihydrotestosterone (DHT) and 5AR were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). The antibody to intercellular cell adhesion molecules (ICAM) was purchased from Abcam, Inc. (Cambridge, Cambs, UK). The antibodies to protein kinase B (PKB/AKT) and phosphorylation-AKT (p-AKT) were purchased from CST, Inc. (Boston, MA, USA). The antibodies to extracellular signal-regulated kinases (ERK) 1/2, p-ERK1/2, p38 and p-p38 were purchased from Bioworld Technology, Inc. (St. Louis Park, MN, USA). The antibodies to nuclear factor (NF)κB-p65 were purchased from Bioss Biotechnology, Inc. (Beijing, China). All other solvents and chemicals used in the study were of analytical grade and purchased from Sinopharm Chemical Reagent, Co Ltd. (Shanghai, China).2.2. Animals and administration
Adult male Sprague-Dawley (S.D.) rats weighing 200–220 g were obtained from the Experimental Animal Center, Tongji Medical College, Huazhong University of Science and Technology, China (license number: SCXKe-2010-0009). The animals were housed at a controlled room (temperature 22 ± 3 °C and humidity 50 ± 10%) and were kept on a 12 h light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h dark cycle. The rats were fed standard diet and water ad libitum, and acclimated for 7 days before they were used for the study. Five groups were set in this study: the vehicle control group (C), the BPH model group (M), the high dose GSP treated-BPH group (H), the low dose GSP treated-BPH group (L), and the positive finasteride treated-BPH group (P). Eight rats that underwent a sham-operated surgery were set as C group. Other animals underwent surgery to cut off the testicles (castration). After a recovery time of one week, these rats were randomly divided into 4 groups (n = 8) to be the M, P, H and L groups, respectively. Then, the M, P, H and L groups were subcutaneously injected with 10 mg per kg per day testosterone (dissolved in olive oil) for 4 weeks while the C group was injected with the same volume of olive oil. One hour after the injection, the rats of H and L groups were orally treated with 400 and 200 mg per kg per day GSP every day, respectively. Similarly, the rats of P groups were orally treated with 5 mg per kg per day finasteride. The rats of M and C groups were orally treated with the same volume of physiological saline.1,2 The GSP and finasteride were dissolved using physiological saline.
12 h dark cycle. The rats were fed standard diet and water ad libitum, and acclimated for 7 days before they were used for the study. Five groups were set in this study: the vehicle control group (C), the BPH model group (M), the high dose GSP treated-BPH group (H), the low dose GSP treated-BPH group (L), and the positive finasteride treated-BPH group (P). Eight rats that underwent a sham-operated surgery were set as C group. Other animals underwent surgery to cut off the testicles (castration). After a recovery time of one week, these rats were randomly divided into 4 groups (n = 8) to be the M, P, H and L groups, respectively. Then, the M, P, H and L groups were subcutaneously injected with 10 mg per kg per day testosterone (dissolved in olive oil) for 4 weeks while the C group was injected with the same volume of olive oil. One hour after the injection, the rats of H and L groups were orally treated with 400 and 200 mg per kg per day GSP every day, respectively. Similarly, the rats of P groups were orally treated with 5 mg per kg per day finasteride. The rats of M and C groups were orally treated with the same volume of physiological saline.1,2 The GSP and finasteride were dissolved using physiological saline.
At the end of the experimental period, the plasma samples were prepared and stored at −80 °C until the analysis of PACP, DHT and 5AR. The prostatic lateral lobe samples were rapidly removed, cleaned with PBS to remove residual blood, blotted dry and weighed for the analysis of prostatic index (PI). PI was calculated by the following equation: PI = prostate weight/body weight. Then, the tissue was excised and one section was stored at −80 °C for the subsequent western blot assay (such as, the prostatic expressions of ERK1/2, p-ERK1/2, p38, p-p38, AKT, p-AKT, NFκB and ICAM). Another section of the tissue was fixed in 4% paraformaldehyde for 3 days. Then, they were embedded in paraffin and serially sectioned for the haematoxylin–eosin staining. Additionally, immunohistochemical analysis for the prostatic expressions of growth factors was performed using deparaffinized prostate sections. The sections were immersed in freshly prepared 2% H2O2 at 37 °C for 10 min and blocked with 5% goat serum for 10 min. Then, a primary antibody (anti-vascular endothelial growth factor (VEGF), transforming growth factor-β1 (TGF-β1) or basic fibroblast growth factor (bFGF)) was added and incubated at 37 °C for 1 h. After being washed with PBS, the sections were treated with the secondary antibody conjugated with horseradish peroxidase at 37 °C for 10 min. Then, they were immersed in diaminobenzidine for 3 min. The hematoxylin-stained sections were dehydrated by ethanol. The negative control was performed by omitting the primary antibody. Stained areas of the sections were visualized using an optical microscope at ×400 magnification and recorded using MOTIC software. The rest of the sections were made into tissue homogenate in 10 volumes of ice cold physiological saline and then stored at −80 °C for the subsequent assay of prostatic levels of SOD, GPx, MDA, TNF-α, IL-1β, IL-6, and COX-2.
All the animal experiments were performed in compliance with the Chinese legislation on the use and care of laboratory animals. The study was approved by the Institutional Animal Care and Use Committee at Tongji Medical College, Huazhong University of Science and Technology (Approval number: S276).
2.3. Biochemical analysis
The level of plasma PACP was assayed using a commercially available kit. All the procedures were performed according to the manufacturer's instructions and the result was expressed as U l−1. The contents of prostatic TNF-α, IL-1β, IL-6 and COX-2 were measured to evaluate the inflammatory responses of the prostate. They were detected by specific ELISA according to the manufacturer's instructions. The levels of TNF-α, IL-1β, IL-6 and COX-2 were expressed as pg ml−1. The plasma levels of DHT and 5AR were also determined using ELISA methods. The results were expressed as nmol l−1 and U l−1, respectively. The prostatic level of oxidative stress was assessed by measuring the levels of SOD, GPx, and MDA according to the reported methods using commercially available kits.2,7 The results of MDA, SOD and GPx were expressed as nmol per mg protein, U per mg protein and U per mg protein, respectively. Protein concentration of each prostate sample was determined using a BCA kit. Bovine serum albumin was used as the standard.2.4. Western blot analysis
The prostatic expressions of ERK1/2, p-ERK1/2, p38, p-p38, AKT, p-AKT, NFκB-p65 and ICAM were evaluated by western blot analysis. In brief, the tissue samples were ground in liquid nitrogen and the total protein was extracted using a protein extraction kit. Protein concentration was determined using the BCA kit. Protein samples (50 μg) were separated by 12% SDS-polyacrylamide gel electrophoresis and then transferred to a PVDF membrane (Roche Diagnostics Corporation, Indianapolis, IN, USA) by electrophoretic transfer (Bio-Rad Laboratories, Inc. Hercules, CA, USA). Transferred membranes were blocked for 1 h at room temperature with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20 (TBST), and then incubated overnight at 4 °C with different primary antibodies (ERK1/2, p-ERK1/2, p38, p-p38, AKT, p-AKT, NFκB-p65 or ICAM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000)). After three washes with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies in TBST with 3% nonfat milk for 1 h at room temperature. Immunoblots were developed on films using the enhanced chemiluminescence technique (Super Signal West Pico; Pierce Biotechnology, Rockford, IL, USA). Quantification of bands was determined by integrated optical density (IOD) analysis using Gel-Pro Analyzer software. The data were normalized using β-actin (1
1000)). After three washes with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies in TBST with 3% nonfat milk for 1 h at room temperature. Immunoblots were developed on films using the enhanced chemiluminescence technique (Super Signal West Pico; Pierce Biotechnology, Rockford, IL, USA). Quantification of bands was determined by integrated optical density (IOD) analysis using Gel-Pro Analyzer software. The data were normalized using β-actin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) as an internal control.
000) as an internal control.
2.5. Statistical analysis
The values were presented as mean ± S.D. Results were analyzed statistically by one-way ANOVA followed by Tukey's multiple comparison using SPSS 11.5. Differences were considered as significant at p < 0.05. Figures were made using Origin 6.0 Software.3. Results
3.1. Biochemical analysis
Effects of GSP on the levels of PI, PACP, DHT and 5AR in testosterone-induced BPH rats are shown in Fig. 1. Testosterone injection led to significant enhancements in the level of PI (Fig. 1A) and the activity of plasma PACP (Fig. 1B) when compared to the vehicle control. GSP produced marked (p < 0.05) effects on decreasing the activity of PACP and the level of PI. Especially, 400 mg per kg per day GSP exerted more effective activity than the positive control (p < 0.05). Similarly, testosterone injection resulted in much higher plasma levels of DHT (Fig. 1C) and 5AR (Fig. 1D). GSP treatment obviously decreased the levels of DHT and 5AR.As indicated in Fig. 2, the rats in the model group express an obvious chronic inflammation performance. The levels of IL-1β (Fig. 2A), IL-6 (Fig. 2B), COX-2 (Fig. 2C) and TNF-α (Fig. 2D) in the rats of BPH model group were significantly higher than the vehicle control (p < 0.05). Four weeks of treatment with 400 or 200 mg per kg per day GSP alleviated the change. Compared to those in the model group, the levels of IL-1β, IL-6, COX-2 and TNF-α were significantly decreased (p < 0.05), and being treated with 400 mg per kg per day GSP for 4 weeks, led to lower levels of these inflammatory cytokines than the positive control (p < 0.05).
Fig. 3 describes the effects of GSP on modulating the levels of prostatic oxidative stress. The levels of SOD (Fig. 3A) and GPx (Fig. 3B) in both GSP treated groups were obviously enhanced when compared to the model control, as well as the levels of MDA (Fig. 3C) being significantly decreased. MDA level is a common indicator for oxidative stress. It can be seen from Fig. 3C that 400 mg per kg per day GSP inhibited the levels of prostatic oxidative stress to a similar extent with the positive control (p > 0.05). These results suggested that GSP could normalize the activities of antioxidant enzymes and inhibit the prostatic level of oxidative stress.
3.2. Histological and immunohistochemical analysis
As can be seen from Fig. 4, the histological analysis shows that the prostate of the vehicle control group is normal in size without any abnormal phenomenon in the morphological structure by visual observation. By contrast, the prostatic epithelial height and the stromal spaces of the BPH model group were obviously increased. Additionally, the papillary fronds of the prostate protruded into the gland cavities and resulted in decreased glandular luminal area. GSP treatment inhibited the changes in morphology. Larger glandular luminal area, decreased extent of the epithelial cells expansion and minor stromal hyperplasia were observed. The expressions of VEGF, bFGF and TGF-β1 in the rat prostate were examined through immunohistochemical analysis. VEGF and bFGF were rarely expressed in normal prostatic epithelial cells. Notably increased expression of VEGF and bFGF was found in the BPH model group. Conversely, the expression of prostatic TGF-β1 was reduced in the rats of BPH model group when compared to the vehicle control group. Administration of GSP suppressed the testosterone-induced diminishment in prostatic TGF-β1 expression as well as attenuated the over-expressions of VEGF and bFGF when compared to the model group.3.3. Western blot analysis
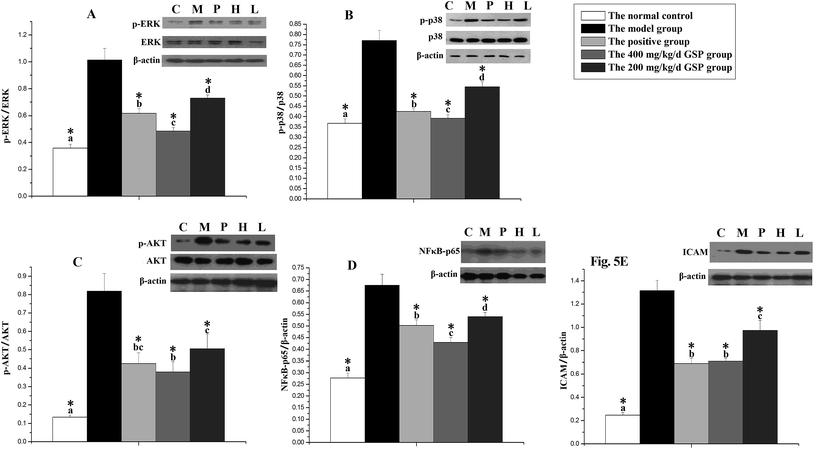
Fig. 5 represents the effects of GSP on regulating the prostatic expressions of ERK1/2, p-ERK1/2, p38, p-p38, AKT, p-AKT, NFκB-p65 and ICAM. Testosterone injection obviously increased the phosphorylation levels of ERK1/2 (Fig. 5A), p38 (Fig. 5B) and AKT (Fig. 5C) as well as enhanced the expressions of p65 (Fig. 5D) and ICAM (Fig. 5E) when compared to the vehicle control. Four weeks of treatment with GSP (200 or 400 mg per kg per day) significantly decreased the levels of p-ERK1/2, p-p38, p-AKT, NFκB-p65 and ICAM compared to those in the untreated model control (p < 0.05). 400 mg per kg per day GSP contributed to noticeably lower prostatic levels of p-ERK1/2, p-p38, p-AKT, NFκB-p65 and ICAM compared to 200 mg per kg per day GSP (p < 0.05).4. Discussion
The testosterone-induced experimental BPH model in castrated rats was used in this study to investigate the potential prostatic protective nature of GSP. PACP is a member of the acid phosphatases and is secreted by prostate. The plasma level of PACP obviously enhances accompanied by a significant increase of the weight of the prostate in the BPH model.7,18 This is in line with our study. Four weeks injection of testosterone resulted in much enhanced levels of PI (Fig. 1A) and PACP (Fig. 1B) in rats. Both GSP and finasteride significantly ameliorated the prostate injury and inhibited the increases of PACP and PI when compared to the testosterone model. The overall results indicated that GSP had the potential anti-BPH nature.Although the exact pathology and etiology of BPH are still not clear, a major agreement about the important role of androgen in prostate growth has been achieved. Many researchers believe that the progression of BPH is mediated by DHT, which is synthesized by 5AR.5,12 DHT can modulate the prostatic balance of proliferation and apoptosis in at least two manners. On one hand, DHT indirectly stimulates the secretion of several growth factors from human prostatic stroma in an androgen-dependent method.19 On the other hand, DHT also controls the release of other inflammatory factors such as IL-1, IL-6 and TNF-α.1,4,20
Among the family of growth factors, bFGF is a polypeptide growth factor having functions of modulating fiber cell growth and regulating the structure of the extracellular matrix during the formation of BPH via exhibiting mitogen and angiogenic activities.1,21 The concentration of bFGF is higher in hyperplastic tissues when compared to normal prostate tissues.22 TGF-β1 is another important growth factor in BPH and regulates the proliferation and differentiation of stromal. In fact, TGF-β1 is the only known growth factor that can suppress tissue proliferation and induce cell apoptosis.1,23 The increased expression of TGF-β1 contributes to inducing the apoptotic death of androgen-dependent prostatic epithelial cells even in the presence of physiological levels of androgens.24 Chronic inflammatory state has become evident as a major factor in BPH progression. As soluble signaling molecules, these cytokines act as the mediators in immune and inflammatory responses.18 Additionally, the chronic inflammation of the prostate creates a pro-angiogenic environment. The angiogenic process of the prostate is stimulated by increased generation of proangiogenic factors including VEGF, bFGF, TNF-α and interleukins. Hormones, cytokines and growth factors stimulate the expression of COX-2. Subsequently, the over expressed COX-2 will lead to the activation of angiogenesis, contribute to the regulation of prostate cell proliferation and exert a link between prostatic inflammation and carcinogenesis.1,3,25 In this study, treatment with 400 or 200 mg per kg per day GSP obviously lowered the DHT level (Fig. 1C) and the 5AR activity (Fig. 1D), and decreased the levels of IL-1β, IL-6, COX-2 and TNF-α (Fig. 2). Similarly, the prostatic expression of TGF-β1 was significantly upregulated as well as the expressions of VEGF and bFGF being inhibited in GSP treated groups when compared to the BPH model group (Fig. 4).
Apart from the chronic inflammatory state, patients with BPH are often characterized by oxidative damage and accompanied with increased MDA level and decreased SOD activity.26 Thus, it is believed that oxidative stress is also implicated in both the initiation and progression of BPH.7 High levels of ROS not only attack DNA directly, but also initiate autocatalytic lipid peroxidation.26 Testosterone increases the production of free radicals and decreases the activities of antioxidant enzymes.20 In the normal case, the generation and elimination of ROS is a dynamic balance. For example, superoxide anion is metabolized into H2O2 by SOD. Then, the generated H2O2 is detoxified into molecular oxygen and H2O by GPx. When the prostatic balance of ROS-antioxidant is destructed, the activities of prostatic antioxidant enzymes (such as SOD and GPx) obviously decrease while the level of MDA significantly enhances.2,7 The polyphenolic substances derived from grape seed have been used as natural antioxidants for a long time and our results also showed that GSP could normalize the activities of antioxidant enzymes and inhibit the prostatic level of oxidative stress in BPH rats (Fig. 3).
It is known that high levels of ROS are related to enhanced expressions of TNF-α and COX-2, and a lower TGF-β1 level.1,3 Furthermore, prostatic inflammation will in turn promote the accumulation of free radicals. NFκB (especially the p65 subunit) regulates growth factors levels, stimulates proinflammatory cytokine gene expressions (like COX-2, TNF-α, IL-1β, and IL-6) and leads to angiogenesis in human prostate cancer cells.9,17,27 Moreover, the growth factors-related angiogenesis signal in BPH can be activated by both of the PI3K/AKT and mitogen-activated protein kinases (MAPK) signaling pathways. Subsequently, these proangiogenic factors will upregulate the expression of ICAM.28 ICAM in turn contributes to the inhibition of apoptosis via activating PI3K/AKT and MAPK pathways.29 At this stage, a vicious cycle initiates. ICAM plays key roles in T lymphocytes activation and cell adhesion. It can be activated by reactive oxygen species (ROS) and NFκB-dependent inflammatory stimuli.30 Especially under chronic inflammatory state, over-expressed ICAM will result in increased membrane permeability and profound tissue injury. Ultimately causing organ dysfunction.31
In brief, NFκB with the functions of regulating cell survival and apoptosis, builds a link among angiogenesis, oxidative stress and inflammatory responses in BPH.32 Thus, it can be demonstrated that the signaling molecule NFκB is a major switch and drives a number of events in BPH. It is already known that the activation of NFκB is regulated by several cellular kinases such as MAPK and AKT.27 MAPK falls into several different subgroups including ERK1/2, ERK5, p38 and Jun N-terminal kinases (JNKs). Among them, ERK1/2 is an important signal transducer for cell survival while p38 contributes to the acquisition of invasion and migrating capabilities.33 Reports show that inhibiting the activity of p38-NFκBp65 signaling can promote apoptosis in prostate cancer cells.34 Similarly, the AKT-NFκB-VEGF pathway regulates lots of cellular processes including cell proliferation and survival, angiogenesis and tissue invasion in human prostate cancer cells.9 Androgens can stimulate the activations of AKT, ERK1/2 and p38.19 Summarily, androgen stimulates the phosphorylations of AKT and MAPK, and subsequently activates the expressions of NFκB and ICAM.30,34
In this study, daily treatment with GSP for 4 weeks obviously inhibited the activity of the androgen-MAPK/AKT-ICAM pathway, normalized the activities of SOD and GPx and regulated the levels of cytokines and growth factors. Ultimately, a lower extent of prostatic disorders was observed. GSP (400 mg per kg per day) exerted similar effects on relieving experimental BPH in rats when compared to the finasteride positive control group. Particularly, GSP (400 mg per kg per day) was even more effective than the positive drug in several aspects (such as in decreasing the generation of proinflammatory factors). In addition, modern medicine also shows that the no-observed-adverse-effect level of grape seed extract in sub-chronic toxicity was up to 1410 mg per kg body weight per day in male rats.35 Taking into account that grapes and grape seeds have been used in Chinese folk medicine for the treatment of prostatitis and BPH for a long time,13 GSP deserves further development for its potential medicinal value in human beings.
5. Conclusions
The overall results suggested that GSP had prostatic protective nature via modulating androgen-MAPK/AKT-ICAM pathway, as well as normalizing antioxidant enzyme activities and regulating the proinflammatory cytokines. Eventually, the prostatic inflammatory response and oxidative stress were alleviated (Fig. 6). | ||
| Fig. 6 Grape seed-derived polyphenols extract (GSP) regulated the possible mechanism of testosterone-induced benign prostatic hyperplasia in castrated rats. | ||
Conflict of interest
The authors declare that there are no conflicts of interest.Abbreviations
| 5AR | 5-α-Reductase |
| bFGF | Basic fibroblast growth factor |
| BPH | Benign prostatic hyperplasia |
| BCA | Bicinchoninic acid |
| COX-2 | Cyclooxygenase-2 |
| DHT | Dihydrotestosterone |
| ELISA | Enzyme linked immunosorbent assay |
| ERK1/2 | Extracellular signal-regulated kinases 1/2 |
| GPx | Glutathione peroxidase |
| GSP | Grape seed-derived polyphenols extract |
| ICAM | Intercellular cell adhesion molecules |
| IL-1β | Interleukin-1β |
| JNKs | Jun N-terminal kinases |
| MDA | Malondialdehyde |
| MAPK | Mitogen-activated protein kinase |
| NFκB | Nuclear factor κB |
| p-AKT | Phosphorylation-AKT |
| PACP | Prostatic acid phosphatase |
| PI | Prostatic index |
| PKB/AKT | Protein kinase B |
| ROS | Reactive oxygen species |
| S.D. | Sprague-Dawley |
| SOD | Superoxide dismutase |
| TGF-β1 | Transforming growth factor-β1 |
| TNF-α | Tumor necrosis factor-α |
| VEGF | Vascular endothelial growth factor |
Acknowledgements
This work was supported by Science Foundation of Tongji Hospital (2013B003).References
- H. Sun, T. J. Li, L. N. Sun, Y. Qiu, B. B. Huang, B. Yi and W. S. Chen, J. Ethnopharmacol., 2008, 115, 203–208 CrossRef PubMed.
- M. I. Ali, H. D. Kondreddi and B. Veeresh, Eur. J. Pharmacol., 2013, 698, 397–403 CrossRef CAS PubMed.
- C. De Nunzio, G. Kramer, M. Marberger, R. Montironi, W. Nelson, F. Schröder, A. Sciarra and A. Tubaro, Eur. Urol., 2011, 60, 106–107 CrossRef CAS PubMed.
- S. H. Li, J. H. Ryu, S. E. Park, Y. S. Cho, J. W. Park, W. J. Lee and Y. S. Chun, J. Nutr. Biochem., 2010, 21, 801–808 CrossRef CAS PubMed.
- B. G. Timms and L. E. Hofkamp, Differentiation, 2011, 82, 173–183 CrossRef CAS PubMed.
- S. D. Chung, C. C. Huang and H. C. Lin, J. Affective Disord., 2011, 134, 404–409 CrossRef PubMed.
- Y. F. Li, L. P. Tang, R. R. He, Z. Xu, Q. Q. He, F. J. Xiang, W. W. Su and H. Kurihara, J. Funct. Foods, 2013, 5, 1357–1365 CrossRef CAS PubMed.
- M. Gasco, L. Villegas, S. Yucra, J. Rubio and G. F. Gonzales, Phytomedicine, 2007, 14, 460–464 CrossRef CAS PubMed.
- H. S. Yu, S. W. Wang, A. C. Chang, H. C. Tai, H. I. Yeh, Y. M. Lin and C. H. Tang, Biochem. Pharmacol., 2014, 87, 243–253 CrossRef CAS PubMed.
- J. A. Macoska, Differentiation, 2011, 82, 253–260 CrossRef CAS PubMed.
- V. Steenkamp, M. C. Gouws, M. Gulumian, E. E. Elgorashi and J. van Staden, J. Ethnopharmacol., 2006, 103, 71–75 CrossRef CAS PubMed.
- S. Martínez Caballero, C. Carricajo Fernández and R. Pérez-Fernández, Fitoterapia, 2004, 75, 187–191 CrossRef PubMed.
- The Editorial Board of China Herb of State Administration of Traditional Chinese Medicine, China Herb, Shanghai Science and Technology Press, Shanghai, 1999 Search PubMed.
- G. Montagut, I. Baiges, J. Valls, X. Terra, J. M. del Bas, X. Vitrac, T. Richard, J. M. Mérillon, L. Arola, M. Blay, C. Bladé, J. Fernández-Larrea, G. Pujadas, J. Salvadó and A. Ardévol, Food Chem., 2009, 116, 265–270 CrossRef CAS PubMed.
- D. Bagchi, A. Garg, R. L. Krohn, M. Bagchi, D. J. Bagchi, J. Balmoori and S. J. Stohs, Gen. Pharmacol., 1998, 30, 771–776 CrossRef CAS.
- C. Ignea, C. M. Dorobantu, C. P. Mintoff, N. Branza-Nichita, M. R. Ladomery, P. Kefalas and V. S. Chedea, Food Chem., 2013, 141, 3967–3976 CrossRef CAS PubMed.
- Y. F. Lei, X. H. Ren, J. L. Chen, D. Liu and J. L. Ruan, J. Funct. Foods, 2014, 7, 416–424 CrossRef CAS PubMed.
- B. Lu, H. Cai, W. Huang, X. Wu, Y. Luo, L. Liu and Y. Zhang, Food Chem., 2011, 124, 1017–1023 CrossRef CAS PubMed.
- A. G. Papatsoris and A. G. Papavassiliou, Trends Mol. Med., 2001, 7, 288–292 CrossRef CAS.
- R. T. Atawia, M. G. Tadros, A. E. Khalifa, H. A. Mosli and A. B. Abdel-Naim, Toxicol. Lett., 2013, 219, 160–169 CrossRef CAS PubMed.
- J. K. Dow and R. W. deVere White, Urology, 2000, 55, 800–806 CrossRef CAS.
- S. Boget, A. Leriche and A. Rovel, Farmaco, 2001, 56, 467–469 CrossRef CAS.
- G. Kramer, D. Mitteregger and M. Marberger, Eur. Urol., 2007, 1, 1202–1216 CrossRef PubMed.
- N. Kyprianou, H. Tu and S. C. Jacobs, Hum. Pathol., 1996, 27, 668–675 CrossRef CAS.
- O. Kranenburg, M. F. Gebbink and E. E. Voest, Biochim. Biophys. Acta, 2004, 1654, 23–37 CAS.
- A. Aydin, Z. Arsova-Sarafinovska, A. Sayal, A. Eken, O. Erdem, K. Erten, Y. Ozqök and A. Dimovski, Clin. Biochem., 2006, 39, 176–179 CrossRef CAS PubMed.
- A. R. Kim, M. S. Lee, T. S. Shin, H. Hua, B. C. Jang, J. S. Choi, D. S. Byun, T. Utsuki, D. Ingram and H. R. Kim, Toxicol. In Vitro, 2011, 25, 1789–1795 CrossRef CAS PubMed.
- C. Francavilla, L. Maddaluno and U. Cavallaro, Semin. Cancer Biol., 2009, 19, 298–309 CrossRef CAS PubMed.
- K. C. Ahn, J. Y. Choi, J. S. Kim, S. G. Hwang, W. J. Kim, J. K. Park and H. D. Um, Biochem. Biophys. Res. Commun., 2013, 441, 507–513 CrossRef CAS PubMed.
- C. W. Kim, T. H. Lee, K. H. Park, S. Y. Choi and J. Kim, FEBS Lett., 2012, 586, 229–234 CrossRef CAS PubMed.
- K. Mehla, S. Balwani, A. Kulshreshtha, D. Nandi, P. Jaisankar and B. Ghosh, J. Ethnopharmacol., 2011, 137, 1345–1352 CrossRef CAS PubMed.
- M. Yan, K. Hardin and E. Ho, J. Nutr. Biochem., 2010, 21, 687–694 CrossRef CAS PubMed.
- S. Wen, Y. Niu, S. O. Lee and C. Chang, Cancer Treat. Rev., 2014, 40, 31–40 CrossRef PubMed.
- B. Gil-Araujo, M. V. Toledo-Lobo, M. Gutiérrez-Salmerón, J. Gutiérrez-Pitalúa, S. Ropero, J. C. Angulo, A. Chiloeches and M. Lasa, Mol. Oncol., 2014, 8, 27–38 CrossRef CAS PubMed.
- J. Yamakoshi, M. Saito, S. Kataoka and M. Kikuchi, Food Chem. Toxicol., 2002, 40, 599–607 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |