A SiO2-coated nanoporous alumina membrane for stable label-free waveguide biosensing
Abstract
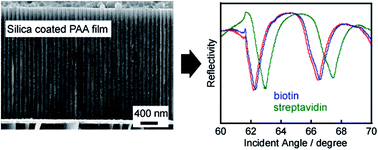
Nanoporous anodic alumina (PAA) has been widely employed in many areas due to its well-ordered and self-organized pore structures. In the present study, we demonstrated two uses of a SiO2 coating: to attach a free-standing PAA membrane to a hydroxylated gold layer for use as a planar optical waveguide sensor and to create a protective layer against etching in aqueous solution by a surface sol–gel (SSG) method. Scanning electron microscopy (SEM) indicates that the as-formed SiO2 layer covers both the inner and outer surfaces of the PAA membrane. Optical waveguide spectroscopy (OWS) measurements show that the apparent waveguide modes were typical for a PAA/Au film with “SiO2 glue”, while only the typical surface plasma resonance curve was observed before the SSG process was performed. Additionally, the SiO2-coated PAA/Au film after five SSG cycles displays greater aqueous stability in the range of pH 2.4 to 7.3 in comparison to the primary PAA membrane, indicating that the SiO2 layer was continuous. The feasibility of label-free optical biosensing was demonstrated by two immunosensor experiments that monitored the adsorption of either bovine serum albumin (BSA) or streptavidin in real time. The application of the SSG method to a thicker PAA film (>2.5 μm) with an organized pore structure for OWS biosensing was demonstrated, and improved sensitivity in good agreement with simulation results was achieved.

 Please wait while we load your content...
Please wait while we load your content...