Synthesis of novel furan-containing tetrafunctional fluorene-based benzoxazine monomer and its high performance thermoset†
Abstract
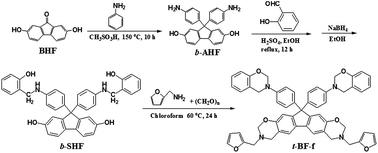
A novel furan-containing tetrafunctional fluorene-based benzoxazine monomer with bisphenol- and diamine-type oxazine rings was successfully prepared. The resulting polybenzoxazine exhibits extremely higher glass transition temperature (440 °C) and better thermal stability than difunctional furan-containing fluorene-based and traditional multifunctional benzoxazine resins.

 Please wait while we load your content...
Please wait while we load your content...