Utilization of hollow kapok fiber for the fabrication of a pH-sensitive superabsorbent composite with improved gel strength and swelling properties
Xiaoning Shiab,
Wenbo Wanga,
Yian Zhenga and
Aiqin Wang*a
aCenter of Eco-material and Green Chemistry, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, P.R. China. E-mail: aqwang@licp.cas.cn; Fax: +86 931 8277088; Tel: +86 931 4968118
bGansu University of Traditional Chinese Medicine, Lanzhou, 730000, P.R. China
First published on 24th September 2014
Abstract
A pretreated kapok fiber (PKF) with a hollow tube structure was introduced into a poly(sodium acrylate) network by a simultaneous free-radical graft copolymerization and crosslinking reaction to fabricate a novel kapok fiber-g-poly(sodium acrylate) (PNaA/PKF) superabsorbent composite. The network characteristics and surface morphologies of the PNaA/PKF superabsorbent composite were investigated by Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM), as well as by determination of mechanical properties, swelling and stimuli responses to salts, and pH. The results showed that the incorporation of a specific amount of PKF not only improved the water absorption, but also the gel content and gel strength. The composite with 10 wt% PKF showed the best water absorption, gel content and gel strength. The swelling kinetics of the composite followed Schott's pseudo-second-order kinetics model, and the swelling rate constant was enhanced 2.63 fold after adding 10 wt% PKF. The swelling and deswelling behaviors in various saline and pH solutions revealed the stimuli-sensitivity of the PNaA/PKF composite to salt concentration, ionic charge and external pH, and a remarkable time-dependent swelling process with an overshooting characteristic was observed in pH 2 solutions.
1 Introduction
Superabsorbent polymers (SAPs) are a kind of lightly crosslinked, hydrophilic functional polymer material with three-dimensional (3D) network structures. They can absorb, swell and retain aqueous solution up to hundreds of times their own mass, and have been extensively applied in many fields, such as agriculture and horticulture,1–3 hygienic products,4,5 wastewater treatment,6–8 drug delivery systems,9–11 biotechnology,12,13 etc. Currently, the design of SAPs from renewable bioresources has become a preferred approach to alleviate the environmental and resource problems resulting from the excessive consumption of petroleum-based raw materials. Thus far, many naturally available materials, such as starch,14,15 chitosan,12,16 cellulose,10,17,18 gum,11,19 protein20,21 and some crop residues,22,23 have been used to produce eco-friendly SAPs through their free-radical graft polymerization with vinyl monomers. It has been confirmed that the properties of SAPs are highly dependent on the structures of the natural polymers. Therefore, the design and development of new SAPs by introducing natural polymers with special structures has become a sustainable research subject for the future.Kapok fiber (KF) is a silky fiber that encloses the seeds of the ceiba tree of the family Bombacaceae. It has a homogeneous hollow tube shape with a wall thickness of ca. 0.8–1.0 μm and is composed of 64% cellulose, 13% lignin, 23% pentosan and some wax cutin on its surface.24 KF is traditionally used as a stuffing, especially for life preservers, bedding, upholstery, and for insulation against sound and heat. Further, it exhibits greater potential as a filter for oily water treatment because of its low density, high porosity, great specific surface area and hydrophobic–oleophilic physicochemical characteristics.25–27 However, the intrinsic hydrophobic properties of raw KF make it quite difficult for it to be dispersed in aqueous solution for graft polymerization. Because NaClO2 can break up some of the phenolic compounds,28 it can be used to treat KF to reduce the lignin content and change the surface properties of KF from hydrophobic to hydrophilic. This moderate modification of KF gives it potential as a matrix to fabricate new materials by a grafting copolymerization reaction. Kang et al. reported the grafting of glycidyl methacrylate onto pretreated KF under irradiation with 60Co gamma rays,28 and confirmed that the surface groups of KF are active and suitable for graft copolymerization. However, there is no related research on the preparation of a KF-based composite with stimuli-responsive properties in aqueous solution by a graft copolymerization reaction.
Acrylic acid (AA) is an important hydrophilic vinyl monomer used for the fabrication of superabsorbent hydrogels. By graft polymerization of partially neutralized AA onto biomacromolecular backbones, a three-dimensional network can be formed and the synthesized superabsorbent composites show improved swelling properties, biodegradabilities and biocompatibilities.23,29–31 In particular, the incorporation of carboxyl groups makes these bioresource-based superabsorbent hydrogels responsive to various stimuli, such as salts and pH, and thus extends their applications to many fields, such as drug release formulations, and adsorption of liquid and metal ions or dyes.8,10,30
Based on the above background, in this study, KF was pretreated by NaClO2 and used to synthesize a superabsorbent composite using green solution polymerization technology. The synthetic process is eco-friendly and no organic solvent was used. The effect of PKF content on the water absorption, gel content, gel strength and time-dependent swelling ratio was investigated systematically. The network structure of the PNaA/PKF superabsorbent hydrogel was characterized by FTIR and SEM, and a possible mechanism was proposed. The swelling behavior of the superabsorbent in various salt and pH solutions was investigated to expand upon its potential application in adsorption, separation, and drug release systems.
2 Experimental section
2.1 Materials
Kapok fiber (KF) was obtained from Shanghai Panda Co. Ltd. (Shanghai, China). Acrylic acid (AA, C.P. grade) was purchased from Shanghai Wulian Chemical Factory (Shanghai, China). Ammonium persulfate (APS, A.R. grade) was purchased from Xi'an Chemical Reagent Factory (Xi'an, China). N,N′-methylene-bis-acrylamide (MBA, A.R. grade) was purchased from Shanghai Chemical Reagent Corp. (Shanghai, China). Sodium Chlorite (NaClO2, C.P. grade) was purchased from Beijing HWRK Chem. Co. Ltd. (Beijing, China). Other agents used are of analytical grade and all solutions were prepared with distilled water.2.2 Pretreatment of KF
The crude KF was smashed with a high-speed grinder for 20 s and then dipped in a 1 wt% NaClO2 solution at 70–80 °C for 1 h. The treated KF was drained and washed with a large amount of distilled water until pH ∼ 7, and then dried in an oven at 100 °C for 24 h to obtain the pretreated kapok fiber (PKF). This treatment process may transform KF from hydrophobic to hydrophilic.282.3 Preparation of the PNaA/PKF superabsorbent composite
A certain amount of PKF, 30 mL of distilled water and 7.2 g of AA (firstly neutralized with 8.8 mL of 8 mol L−1 NaOH solution in an ice bath) were added into a 250 mL four-necked flask equipped with a mechanical stirrer, a condenser, a thermometer and a nitrogen line. The mixture was heated to 60 °C with an oil bath and stirred under nitrogen purging for 30 min to remove the dissolved oxygen. Afterward, 10 mL of the aqueous solution containing 100 mg of APS and 14.4 mg of MBA was added, and the reactor was slowly heated to 70 °C and kept at this temperature for 3 h to complete the polymerization. The resulting product was dried in an oven at 70 °C for 72 h. The dried product was ground into particles and passed through a 40–80 mesh sieve.2.4 Measurement of gel content and gel strength
In order to determine the gel content, 0.10 g (M1) of dry sample was soaked in 300 mL of distilled water for 72 h, and then filtered. The extracted swollen gels were firstly dewatered by anhydrous ethanol and then dried for 12 h at 70 °C. The dried samples were reweighed (M2) and the gel content (Gel%) was calculated by eqn (1):| Gel% = M2/M1 × 100% | (1) |
The gel strength was measured on a Physica MCR 301 Rheometer (Germany) by a rheological method, and expressed as the rheological curves of storage modulus (G′, Pa) versus angular frequency (ω, rad s−1). The constant deformation strain is 0.5% and the angular frequency (ω) is in the range of 0.1–100 rad s−1.
2.5 Measurement of equilibrium water absorption and swelling kinetics
0.050 g of the dried samples were immersed in 200 mL of distilled water or other swelling media at room temperature for 3 h to reach swelling equilibrium. The swollen hydrogels were separated from the unabsorbed water by a 100-mesh sieve, and then were gently dabbed with filter paper to remove the residual water on the surface. The equilibrium water absorption (Qeq, g g−1) was calculated by eqn (2).| Qeq = (Ms − Md)/Ms | (2) |
Here, Md and Ms are the masses of the dried sample (g) and the swollen hydrogel (g), respectively. The measurements were repeated three times to obtain a mean value of Qeq, and the ±SD value was less than 3%.
The swelling kinetics were measured by the following procedure: 0.050 g of each of the samples was soaked in 200 mL of solution, and the swollen gels were filtered out using a sieve at different time intervals (1, 3, 5, 7, 10, 15, 20, 30, 60 and 120 min), the swelling rate (Qt) at a given time t was measured by weighing the swollen (Mst) and dry (Md) samples and then calculated using eqn (2).
The deswelling kinetics of the swollen superabsorbent in different salt solutions were also measured using the above procedure. The fully swollen gels were soaked in 200 mL of salt solution. At the set time intervals, the samples were taken out of the salt solution and weighed after removing the residual fluids. Water retention (WR) in the superabsorbent was calculated by eqn (3):
| WR = (Mt − Md)/Ms × 100 | (3) |
2.6 Characterization
FTIR spectroscopy was recorded on a Nicolet NEXUS FTIR spectrometer in the 4000–400 cm−1 region, using a KBr pellet. The morphologies of the PNaA/PKF composites were examined using a S-4800 scanning electron microscope after coating the samples with gold film.3 Results and discussion
3.1 Synthesis of the superabsorbent composite
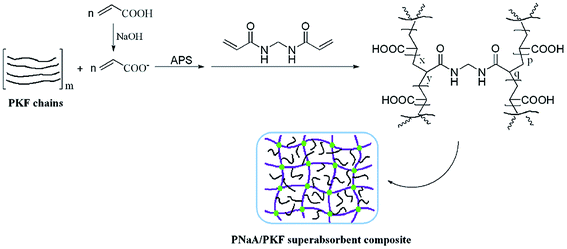
The free-radical graft polymerisation mechanism of polysaccharides, such as chitosan, guar gum and cellulose, with AA was described elsewhere.16,29,31 According to the literature, a proposed mechanism for the grafting and chemical crosslinking of the PNaA/PKF superabsorbent is depicted in Scheme 1. Firstly, for the purpose of enhancing electrostatic repulsion and expanding the polymer network, AA was treated with NaOH solution to convert the COOH groups to COO− groups. Then, the PKF was dispersed in the partially neutralized AA solution, and the thermal decomposition of APS generated sulfate anion radicals, which extracted the hydrogen atoms from the hydroxyl groups of the PKF to form alkoxy radicals, resulting in active centers on the PKF to initiate radical graft copolymerization of AA with the PKF. In the presence of the crosslinker MBA, the end vinyl groups of MBA crosslinked the polymer chains and finally formed the 3D polymer network of PNaA/PKF. The PKF fiber was interpenetrated and entangled within the 3D network of PNaA.3.2 FTIR spectroscopy analysis
The FTIR spectra of the PKF, PNaA, the physical mixture of PKF and PNaA, and the PNaA/PKF (10%) superabsorbent composite are shown in Fig. 1. The PKF shows a broad band at 3412 cm−1 due to the stretching vibration of the O–H groups. The band at 2917 cm−1 is the C–H stretching vibration of the methyl and methylene groups in cellulose.27 The relatively intense band at 1742 cm−1 is assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration from the carbonyl group, and the bands in the region of 1598–1426 cm−1 are ascribed to the skeletal C
O stretching vibration from the carbonyl group, and the bands in the region of 1598–1426 cm−1 are ascribed to the skeletal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of the residual aromatic ring in the PKF.28 The characteristic absorption band at 1055 cm−1 is attributed to the C–O stretching vibration of cellulose, which are obviously weakened after reaction (Fig. 1d). However, it can still be observed in the spectrum of the physical mixture of PKF and PNaA with almost no change in intensity (Fig. 1c). In Fig. 1d, new bands at 1731, 1571, and 1457 cm−1 are related to the stretching vibration of C
C stretching vibrations of the residual aromatic ring in the PKF.28 The characteristic absorption band at 1055 cm−1 is attributed to the C–O stretching vibration of cellulose, which are obviously weakened after reaction (Fig. 1d). However, it can still be observed in the spectrum of the physical mixture of PKF and PNaA with almost no change in intensity (Fig. 1c). In Fig. 1d, new bands at 1731, 1571, and 1457 cm−1 are related to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and the asymmetrical stretching vibration and symmetrical stretching vibration of –COO−, respectively,29,30 which indicate the presence of –COO− groups in the composite network. These results indicate that AA monomers were actually grafted onto PKF.
O, and the asymmetrical stretching vibration and symmetrical stretching vibration of –COO−, respectively,29,30 which indicate the presence of –COO− groups in the composite network. These results indicate that AA monomers were actually grafted onto PKF.
 | ||
| Fig. 1 FTIR spectra of (a) PKF, (b) PNaA, (c) the physical mixture of PKF and PNaA and (d) PNaA/PKF (10 wt%). | ||
3.3 Morphological analysis
SEM micrographs of the crosslinked PNaA, the PKF and the PNaA/PKF superabsorbent with 10 wt% PKF are shown in Fig. 2. The tube structure of KF was still retained after NaClO2 treatment, but the outer surface became uneven relative to that of the untreated sample (Fig. 2b). In addition, the crosslinked PNaA clearly displayed a tight and smooth surface (Fig. 2a), whereas the PNaA/PKF superabsorbent containing 10 wt% PKF showed an undulant and coarse surface (Fig. 2c). This comparatively rough surface is convenient for increasing the surface area and capillary effect, which will facilitate the diffusion of water into the polymeric network, and increasing the equilibrium water absorption and swelling rate.323.4 Effect of PKF content on equilibrium water absorption, gel content and gel strength
The effect of PKF content on the equilibrium water absorption, gel content and storage modulus (G') is shown in Table 1 and Fig. 3. It is obvious that the equilibrium water absorption, the gel content and the G′ values of the PNaA/PKF superabsorbents increased with increasing the amount of PKF to 10 wt% and then decreased. The natural KF is significantly hydrophobic and does not get wet with water. After treatment with NaClO2, the surface properties of KF changed from hydrophobic to hydrophilic owing to delignification and removal of oily compounds.28 Thus, like other polysaccharides, –OH groups on the surface of PKF can graft with vinyl monomer under free-radical conditions.29 When the content of PKF was lower, the radical active sites on PKF backbones were distributed densely. After some vinyl monomers had been grafted on the active sites, the formed graft chains generated steric hindrance and prevented the grafting of other monomers in these grafting sites. The above process is beneficial for the formation of multiple branched side chains and the regulation of the 3D network structures of the superabsorbents.31 As a result, the equilibrium water absorptions, gel contents and gel strengths of the composites increased with increasing the amount of PKF to 10 wt%. However, when the content of PKF exceeded 10 wt%, the excessive PKF made it difficult to form a uniform dispersion in the reaction medium owing to its insolubility in aqueous solution, and the initiation efficiency decreased as the reactive sites on the PKF couldn't be adequately formed. Consequently, both the grafting efficiency and the molecular weights of the grafted PNaA chains decreased. Further, the excessive PKF was mainly physically filled in the network voids. This PKF physically filling the voids prevented the crosslinking reaction on multiple functional groups of the same chain, resulting in more dangling chains and an increased content of the water-soluble component.17,31,33 Thus, the equilibrium water absorption, gel content and gel strength were decreased. It deserves to be pointed out that the gel content of the PNaA/PKF superabsorbent reached 95.7%. As reported previously, the gel contents of collagen-based hydrogel,34 alginate-based hydrogel,35 and starch-based hydrogel36 are 67%, 80% and 81%, respectively, all of which are lower than that of PNaA/PKF. This indicates that the PKF enhances the gel content of the superabsorbent.| PKF content (wt%) | Qeq (g g−1) | SD | Gel% | SD |
|---|---|---|---|---|
| 0 | 195 | 1.1314 | 84.8 | 0.4431 |
| 5 | 270 | 1.9799 | 88.2 | 0.6881 |
| 10 | 356 | 0.1414 | 95.7 | 0.8045 |
| 15 | 292 | 1.4142 | 86.6 | 0.6205 |
| 20 | 247 | 0.9899 | 81.5 | 0.6364 |
3.5 Effect of PKF content on swelling kinetics
Generally, the swelling of the superabsorbent is dependent on the solvent diffusion and the polymer chain relaxation. Fig. 4a shows the time-dependent water absorption of PNaA/PKF superabsorbents with various amounts of PKF in distilled water. The swelling kinetics curves were characterized by two regions: an initial linear step (within 600 s) and an asymptotical plateau towards the equilibrium water absorption (Qeq). For evaluating the kinetic swelling behavior of the superabsorbents, the swelling parameters, including the initial swelling rate constant kis and theoretical equilibrium water absorption Q∞, were determined by fitting the experimental data to Schott's pseudo-second-order kinetics model, represented as:37
 | (4) |
 | ||
| Fig. 4 (a) Time-dependent water absorption of the PNaA/PKF superabsorbent composite in distilled water, and (b) the plots of t/Qt versus t. | ||
| PKF content (wt%) | Q∞ (g g−1) | kis (gg−1 s−1) | R2 |
|---|---|---|---|
| 0 | 196 | 1.8471 | 0.9994 |
| 5 | 271 | 3.0450 | 0.9996 |
| 10 | 360 | 4.8556 | 0.9998 |
| 15 | 292 | 4.2189 | 0.9992 |
| 20 | 248 | 3.1845 | 0.9996 |
As depicted in Table 2, the initial swelling rate, kis, clearly increases on incorporating the PKF and the superabsorbent with 10 wt% PKF shows a maximum kis. Generally, the initial swelling rate is related to the relaxation rate of the polymer chains in networks, which is affected by the degree of crosslinking, the rigidity/flexibility and the hydrophobicity/hydrophilicity.38 The enhancement of the kis values of the PNaA/PKF superabsorbents might result from the increased flexibility of polymeric networks due to the presence of PKF. This is interpreted as evidence that PKF participates in the formation of the 3D network and that the hollow PKF will contribute to the fabrication of a micro-pipeline structure in the polymer network. This structure makes the relaxation of polymer chains and diffusion of water molecules into the polymer network easier. As a result, the swelling rate of the superabsorbent is improved. However, as discussed in Table 1, excessive PKF (>10 wt%) mainly physically fills the polymeric network, which not only decreases the gel content and blocks up the network space for the rapid diffusion of water molecules, but also reduces the hydrophilicity of the polymer network, and so the swelling rate decreases.
3.6 Effect of salt solution on the swelling behaviors
In general, the swelling of the polyelectrolyte gel mainly results from the competition between the Donnan osmotic pressure and the elasticity of the gel network.39 Fig. 5a–c shows the swelling behaviors of the PNaA/PKF superabsorbent composites in various concentrations of NaCl, CaCl2 and AlCl3 solutions. The equilibrium water absorption of the PNaA/PKF superabsorbents in various salt solutions is in the order: PKF 10 wt% > PKF 15 wt% > PKF 5 wt% > PKF 20 wt% > PKF 0 wt%, which is in accordance with that in distilled water. Clearly, the amount of PKF contributes to increase the swelling properties, due to improvement of the composite network structure.Fig. 5a–c also shows that the equilibrium water absorption strongly depends on the concentration and “type” of salt swelling medium. The equilibrium water absorption decreases with increasing salt concentration, and the composite network shrinks in counterion electrolyte solutions (e.g., Na+, Ca2+ and Al3+). These shrinking behaviors can be explained by Donnan equilibrium theory:40
 | (5) |
3.7 Effect of external pH on the swelling behaviors
The stimuli-responsive behavior of an ionic hydrogel is extremely important to extend its applications into the biomedical field. The responsive behavior is characterized by the change of swelling volume when it is subject to external stimuli, e.g., pH or salt. Fig. 6a shows the effect of external pH values on the equilibrium water absorption of the PNaA/PKF with various amounts of PKF. Because the ionic strength may also affect the swelling behavior of the superabsorbents,39 the desired basic and acidic pH was only adjusted by NaOH (pH 13.0) and HCl (pH 1.0) solutions. As shown in Fig. 6, the equilibrium water absorption of all samples is lower at pH 2, but rapidly increases and remains almost constant at pH 4–10. The further increase of pH from 11 to 13 causes a steep decrease of water absorption. This is related to the change of charge repulsion and osmotic pressure in the polyelectrolyte network, which is dependent on the pH values of the swelling medium. At lower pH values, some –COO− groups are gradually protonated, and the electrostatic repulsive forces between the negatively charged –COO− groups are reduced and the hydrogen-bonding interactions between –COOH groups are strengthened. Consequently, the expansion of the polymer network is limited and the water absorption is lower. At higher pH values (4–10), most of the carboxylate groups are ionized, which helps to expand the gel network and improve the water absorption. With further increasing the pH (>10) of the external solution, the concentration of counter-ions (Na+) with high mobility is increased, which leads to a reduction of the Donnan osmotic pressure in the gel structure40 and thus decreases the water absorption. It can also be noted from Fig. 6a that all the PNaA/PKF superabsorbent composites show relatively higher water absorption in each pH value than the PNaA hydrogel. This is mainly because the grafted hollow PKF makes the PNaA network looser and allows it to expand more easily. | ||
| Fig. 6 (a) Water absorption of PNaA/PKF superabsorbents with different amounts of PKF in pH 2–13 solutions, and (b) dynamic swelling curves of the PNaA/PKF (10 wt%) in pH 2, 7 and 12 solutions. | ||
Fig. 6b shows the time-dependent swelling behavior of the PNaA/PKF (PKF 10 wt%) superabsorbent composite in pH 2, 7 and 12 solutions. The water absorption of the sample in pH 7 and 12 solutions increases with prolonging the swelling time until reaching equilibrium, which is similar to the swelling kinetics in distilled water. The decreased equilibrium water absorption in pH 12 solution is due to the charge screening effect. However, in pH 2 solution, the water absorption firstly increases within 5 min, reaches a maximum swelling (77 g g−1), and then gradually decreases to a constant value (17 g g−1). This anomalous time-dependent swelling behavior is known as the “overshooting effect”,43 which is depicted in Scheme 2. When the composite makes contact with the swelling medium, hydrogen-bonding interaction forces are established between the carboxyl ions and water molecules, and water molecules enter the hydrogel network to expand it. This is a swelling process. Afterward, the gradual protonation of the carboxyl ions in acid medium generates numerous –COOH groups, which may form cooperative physical cross-linking by the hydrogen-bonding interactions between them, and then make the network shrink to exclude the absorbed water. This is the deswelling process.
4 Conclusion
A novel PNaA/PKF superabsorbent composite was successfully prepared by the graft copolymerization of a PKF and partially neutralized AA in aqueous solution. FTIR spectra show that the PNaA chains were grafted onto the –OH groups of the PKF. SEM observations confirm that the hollow PKF was interpenetrated and embedded within the hydrogel network and formed a homogeneous composite. The content of the PKF has greater influence on the water absorption, gel content, gel strength and time-dependent swelling behaviors, and the incorporation of 10 wt% of PKF can improve the gel strength and swelling properties to an optimum. The impact of salts on water absorption and time-dependent water retention of the PNaA/PKF superabsorbent is relative to the concentration of salt solution and the valency of the cations. The swelling ratio of the composites decreased with an increase of the salt concentration. The time-dependent deswelling rate in 15 mmol L−1 of NaCl, CaCl2 and AlCl3 solutions is in the order: Al3+ > Ca2+ > Na+. Furthermore, the water absorption of the PNaA/PKF superabsorbent is sensitive to external pH values. The time-dependent swelling in pH 2 solution exhibits a remarkable “overshooting effect”. This confirms that ionic repulsion between electriferous groups that exists in the polymeric network, can be modulated by altering the external pH, and becomes the main driving force for the change in water absorption.Acknowledgements
The authors gratefully acknowledge joint support of this research by the National Natural Science Foundation of China (no. 21107116 and 21377135).References
- L. Wu, M. Z. Liu and R. Liang, Bioresour. Technol., 2008, 99, 547 CrossRef CAS PubMed.
- B. L. Ni, M. Z. Liu, S. Y. Lu, L. H. Xie and Y. F. Wang, J. Agric. Food Chem., 2010, 58, 12373 CrossRef CAS PubMed.
- Z. H. Ma, Q. Li, Q. Y. Yue, B. Y. Gao, X. Xu and Q. Zhong, Bioresour. Technol., 2011, 102, 2853 CrossRef CAS PubMed.
- O. Ozay, S. Ekici, Y. Baran, N. Aktas and N. Sahiner, Water Res., 2009, 43, 4403 CrossRef CAS PubMed.
- K. Kabiri, S. Hesarian, A. Jamshidi, M. J. Zohuriaan-Mehr, H. Boohendi, M. R. Poorheravi, S. A. Hashemi and F. Ahmad-Khanbeigi, J. Appl. Polym. Sci., 2011, 120, 2716 CrossRef CAS.
- Y. Bulut, G. Akçay, D. Elma and I. E. Serhath, J. Hazard. Mater., 2009, 171, 717 CrossRef CAS PubMed.
- S. Chatterijee, T. Chatterjee and S. H. Woo, Bioresour. Technol., 2010, 101, 3853 CrossRef PubMed.
- Y. Ohsedo, M. Miyamoto, M. Oono, K. Shikii and A. Tanaka, RSC Adv., 2013, 3, 3844 RSC.
- C. H. Liu, Y. Q. Chen and J. G. Chen, Carbohydr. Polym., 2010, 79, 500 CrossRef CAS PubMed.
- T. S. Anirudlhan, A. R. Tharun and S. R. Rejeena, Ind. Eng. Chem. Res., 2011, 50, 1866 CrossRef.
- S. Baljit and S. Nisha, Colloids Surf., B, 2011, 82, 325 CrossRef PubMed.
- L. D. Zhang, S. D. Zheng, D. E. Kang, J. Y. Shin, H. Suh and I. Kim, RSC Adv., 2013, 3, 4692 RSC.
- I. L. Kim, R. L. Mauck and J. A. Burdick, Biomaterials, 2011, 32, 8871 Search PubMed.
- M. D. Teli and N. G. Waghmare, Carbohydr. Polym., 2010, 81, 695 CrossRef CAS PubMed.
- J. Kuang, K. Y. Yuk and K. M. Huh, Carbohydr. Polym., 2011, 83, 284 CrossRef CAS PubMed.
- J. P. Zhang, L. Wang and A. Q. Wang, Ind. Eng. Chem. Res., 2007, 46, 2497 CrossRef CAS.
- L. Mikael, S. Mats and L. Anette, Soft Matter, 2010, 8, 207 CrossRef.
- C. D. Delhom, L. A. White-Ghoorahoo and S. S. Pang, Composites, Part B, 2010, 41, 475 CrossRef PubMed.
- W. B. Wang, J. P. Zhang and A. Q. Wang, Appl. Clay Sci., 2009, 46, 21 CrossRef CAS PubMed.
- M. J. Zohuriaan-Mehr, A. Pourjavadi, H. Salimi and M. Kurdtabar, Polym. Adv. Technol., 2009, 20, 655 CrossRef CAS.
- G. B. Marandi, G. R. Mandavinia and S. Ghafary, J. Appl. Polym. Sci., 2011, 120, 1170 CrossRef.
- M. D. Teli and N. G. Waghmare, Carbohydr. Polym., 2009, 78, 492 CrossRef CAS PubMed.
- X. Y. Ma, R. L. Wei, J. W. Cheng, J. P. Cai and J. Zhou, Carbohydr. Polym., 2011, 86, 313 CrossRef CAS PubMed.
- K. Hori, M. E. Flavier, S. Kuga, T. B. T. Lam and K. Iiyama, J. Wood Sci., 2000, 46, 401 CrossRef CAS.
- E. Khan, W. Virojnagud and T. Ratpukdi, Chemosphere, 2004, 57, 681 CrossRef CAS PubMed.
- X. F. Huang and T. Lim, Desalination, 2006, 190, 295 CrossRef CAS PubMed.
- M. A. Abdullah, A. U. Rahmah and Z. Man, J. Hazard. Mater., 2010, 177, 683 CrossRef CAS PubMed.
- P. H. Kang, J. P. Jeun, B. Y. Chung, J. S. Kim and Y. C. Nho, J. Ind. Eng. Chem., 2007, 13, 956 CAS.
- X. N. Shi, W. B. Wang and A. Q. Wang, Colloids Surf., B, 2011, 88, 279 CrossRef CAS PubMed.
- X. W. Peng, J. L. Ren, L. X. Zhong, F. Peng and R. C. Sun, J. Agric. Food Chem., 2011, 59, 8208 CrossRef CAS PubMed.
- W. B. Wang, J. Wang, Y. R. Kang and A. Q. Wang, Composites, Part B, 2011, 42, 809 CrossRef PubMed.
- A. Sannino and L. Nicolais, Polymer, 2005, 46, 4676 CrossRef CAS PubMed.
- I. M. El-Sheribiny and H. D. C. Smyth, Carbohydr. Res., 2010, 345, 2004 CrossRef PubMed.
- A. Pourjavadi, H. Salimi and M. Kurdtabar, J. Appl. Polym. Sci., 2007, 106, 2371 CrossRef CAS.
- H. Ghasemzadeh and F. Ghanaat, J. Polym. Res., 2014, 21, 355 CrossRef PubMed.
- H. L. Abdel-Mohdy, E. A. Hegazy and H. A. Abdel-Rehim, J. Macromol. Sci., Part A: Pure Appl. Chem., 2006, 43, 1051–1063 CrossRef.
- H. Schott, J. Macromol. Sci., Part B: Phys., 1992, 31, 1 CrossRef CAS.
- Y. Zhao, T. Tan and T. Kinoshita, Polymer, 2009, 48, 666 Search PubMed.
- F. Rosa, J. Bordado and M. Casquilho, Polymer, 2002, 43, 63 CrossRef CAS.
- F. Rosa, J. Bordado and M. Casquilho, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 505 CrossRef CAS.
- J. B. Sokoloff, Soft Matter, 2010, 6, 3856 RSC.
- J. P. Zhang, R. F. Liu, A. Li and A. Q. Wang, Polym. Adv. Technol., 2006, 17, 12 CrossRef CAS.
- Y. H. Yin, X. M. Ji, H. Dong, Y. Ying and H. Zheng, Carbohydr. Polym., 2008, 71, 682 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |