A high molecular weight acrylonitrile copolymer prepared by mixed solvent polymerization: I. effect of monomer feed ratios on polymerization and stabilization
Anqi Ju*a,
Yafeng Yana,
Dawei Wangb,
Jun Luoa,
Mingqiao Gea and
Mengjuan Li*a
aCollege of Textile & Clothing, Jiangnan University, Lihu road 1800, 214122 Wuxi, China. E-mail: anqiju@163.com
bThe Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, China. E-mail: wangdw@jiangnan.edu.cn
First published on 19th November 2014
Abstract
A bifunctional comonomer β-methylhydrogen itaconate (MHI) was employed to prepare high molecular weight poly[acrylonitrile-co-(β-methylhydrogen itaconate)] [P(AN-co-MHI)] copolymers by radical polymerization in a mixed solvent of dimethyl sulfoxide/deionized water = 60/40 (wt/wt), which was used as a carbon fiber precursor instead of acrylonitrile terpolymers for improving the stabilization and spinnability simultaneously. The structure and stabilization of P(AN-co-MHI) copolymers with different monomer feed ratios were characterized by elemental analysis (EA), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and differential scanning calorimetry (DSC). The results show that both the polymerization conversion and molecular weight reduce with the increase of MHI content in the feed due to the larger molecular volume of MHI. The monomer reactivity ratios were calculated by Fineman–Ross and Kelen–Tüdõs methods and the reactivity of MHI is higher than that of AN. Two parameters Es = A1618 cm−1/A2244 cm−1 and SI = (I0 − Is)/I0 were defined to evaluate the extent of stabilization and the activation energy (Ea) of the cyclization. The FTIR, XRD and DSC results show that P(AN-co-MHI) copolymers exhibit significantly improved stabilization characteristics than the PAN homopolymer and poly(acrlonitrile–methyl acrylate–acrylic acid) terpolymer, such as a larger extent of stabilization, lower initiation temperature and smaller Ea of cyclization, which is attributed to the ionic initiation of the MHI comonomer and is beneficial for preparing high performance carbon fiber.
1. Introduction
Carbon fiber, an ideal reinforcing material for advanced composite materials, has been extensively applied in high-tech aerospace, defense areas, and civil engineering (e.g., transportation industry, architecture industry, medical industry, and sports equipment).1–3 It has been documented that 90% of the carbon fibers used today are made from a polyarylonitrile (PAN)-based precursor. In recent years, carbon nanofibers, porous carbons, nanocarbons made from acrylonitrile polymers have been extensively studied, and can be used for catalysts,4–6 sensors,7 gas capture8 and supercapacitors.9–12 PAN homopolymer has hardly been used as a precursor for carbon fiber because of its poor spinnability and stabilization such as high stabilization temperature and centralized heat release.13–15 Therefore, a small amount of comonomers are usually incorporated into PAN chains to enhance solubility, spinnability, hydrophilicity and drawability, and especially to promote the stabilization of PAN which plays an important role in the properties of the carbon fiber.16 Generally, acidic comonomers, such as acrylic acid (AA), methacrylic acid (MA) and itaconic acid (IA), are introduced to reduce the initiation temperature of cyclization and improve the stabilization of PAN.17,18 Neutral comonomers like methyl acrylate (MA) and methyl methacrylate (MMA) are used to improve the solubility, drawability and spinnability of PAN.19,20 Many acrylonitrile terpolymers, such as P(AN–MA–IA), P(AN–MMA–IA) and P(AN–MA–AA), are used as precursor to prepare carbon fiber.21 The spinnability of acrylonitrile terpolymers has been improved by the incorporation of neutral comonomers which act as a lubricant. However, the initiation temperature of acrylonitrile terpolymers is almost as high as PAN homopolymer's and the heat release is still centralized, which breaks molecular chains during stabilization.22 The reason is that the reactivity of acidic comonomers is lower than that of neutral comonomers, which results in less acidic comonomers incorporated into the polymer chains during polymerization. In addition, the sequence structure of terpolymer chains is difficult to control. For the above reasons, the acrylontitrile terpolymers are not the best material for carbon fiber precursor. In our previous work a bifunctional comonomer β-methylhydrogen itaconate (MHI) containing carboxyl group and ester group was synthesized to prepare poly[acrylonitrile-co-(β-methylhydrogen itaconate)] [P(AN-co-MHI)] copolymer by solution polymerization, which was used as carbon fiber precursor instead of acrylonitrile terpolymers.23 It was found out that the stabilization and spinnability of PAN were improved effectively by the bifunctional MHI. However, the highest molecular weight of P(AN-co-MHI) copolymer prepared by solution polymerization in our previous work is only 9.85 × 104 g mol−1, which is not beneficial to preparing high performance carbon fiber due to the low molecular weight of P(AN-co-MHI).In order to increase the molecular weight of P(AN-co-MHI), a mixed solutions of dimethyl sulfoxide/deionized water = 60/40 (wt/wt) was used as reaction media to synthesize P(AN-co-MHI) copolymer in this work. The chain transfer constant of deionized water is 0 for radical ∼∼∼AN˙, which can avoid the chain transfer of radical ∼∼∼AN˙ and improve the molecular weight of P(AN-co-MHI) significantly. The copolymerization of AN with different amounts of MHI in the mixed solutions of dimethyl sulfoxide/deionized water were investigated in detail. The effect of monomer feed ratios on polymerization and stabilization were studied by elemental analysis (EA), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and differential scanning calorimetry (DSC) sufficiently.
2. Experimental
2.1 Materials
Acrylonitrile (AN, analytical grade) was purchased from Shanghai Boer Chemical Reagent Co., Ltd. Shanghai, China and distilled twice before polymerization. Azodiisobutyronitrile (AIBN, analytical grade) was commercially provided by Aladdin Industrial Corporation, Shanghai, China and recrystallized twice by methanol. Dimethyl sulfoxide (DMSO, analytical grade) was received from Shanghai Huadong Reagent Company, Shanghai, China and distilled under reduced pressure before use. Itaconic acid (IA), petroleum ether, methanol, benzene and benzoyl chloride (analytical grade) were commercially supplied by Sinopharm Chemical Reagent Co., Ltd. Shanghai, China and used as received.2.2 Synthesis of bifunctional comonomer MHI
To a 100 mL round-bottomed flask was added IA (13.00 g, 100.00 mmol), methanol (14.20 mL, 350.00 mmol) and benzoyl chloride (0.50 mL, 4.30 mmol). The mixture was refluxed at 65 °C for 0.5 h and then cooled to room temperature. The reaction mixture was distilled under reduced pressure to remove excess methanol and then followed by standing to get precipitation. The precipitation was recrystallized from benzene–petroleum ether (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
White crystal, 84.52% yield. IR (KBr) νmax cm−1: 3004, 2955 (C–H), 1726, 1691 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1636 (C
O), 1636 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1237, 1170 (C–O). 1H-NMR (400 Hz, DMSO-d6, RT, TMS) δ ppm: 12.616 (s, 1H, COOH), 6.149 (d, J = 1.20 Hz, 1H, CH2
C), 1237, 1170 (C–O). 1H-NMR (400 Hz, DMSO-d6, RT, TMS) δ ppm: 12.616 (s, 1H, COOH), 6.149 (d, J = 1.20 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 5.763 (d, J = 1.20 Hz, 1H, CH2
), 5.763 (d, J = 1.20 Hz, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 3.580 (s, 3H, OCH3), 3.336 (s, 2H, CH2).
), 3.580 (s, 3H, OCH3), 3.336 (s, 2H, CH2).
2.3 Preparation of P(AN-co-MHI) by radical polymerization in mixed solvents
Copolymerization of AN with different amounts of MHI were carried out in a three-necked flask at 60 °C under nitrogen atmosphere using mixed solvents of DMSO/deionized water = 60/40 (wt/wt) as the reaction medium. In the reaction system, the total monomer concentration was 24 wt%, and the concentration of AIBN was 0.8 wt% on the basis of monomer. Good agitation was used to make sure the heat of polymerization throw off sufficiently and the reaction was terminated after 12 h. The resultant precipitate was filtered and washed with methanol for three times. The isolated precipitate was dried at 75 °C under vacuum to a constant weight. For comparison, PAN homopolymer and poly(acrlonitrile–methyl acrylate–acrylic acid) [P(AN–MA–AA)] with molar feed ratios AN/MA/AA = 98/2/2 was also prepared in this work.In order to determine the monomer reactivity ratios, similar polymerization conditions were used to prepare P(AN-co-MHI) with the mole fraction of MHI in the feed varying from 1 to 5 mol% and the copolymerization was terminated at a low conversion of ≤10%.
2.4 Characterization
Viscosity average molecular weight (Mv) of the acrylonitrile polymers was measured in a (50 ± 0.5) °C water bath using Ubbelolohde viscometer method. The equation is expressed as follows (eqn (1)):| [η] = 2.83 × 10−4Mv0.759 | (1) |
X-ray diffraction (XRD) patterns of power P(AN-co-MHI) copolymers were measured on a Rigaku D/max-2550 diffractometer between 3–50° at the rate of 3° min−1. All the XRD measurements were carried out under the same conditions. The interplanar spacings (d) and crystallite size (Lc) of the crystal were estimated by the Scherrer equation and Bragg equation respectively as follows:25
d = λ/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (2) |
Lc = Kλ/B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (3) |
| CI = Ac/(Ac + Aa) | (4) |
For the stabilization of P(AN-co-MHI), the powder samples were heated at 200 °C in an air oven with a temperature accuracy of 1 °C for 30 minutes.
3. Results and discussion
3.1 Reactivity ratio studies
The molecular weights of MHI and AN are 144 and 53 g mol−1, respectively. According to the mechanism of radical polymerization, the molar fraction of MHI in P(AN-co-MHI) can be calculated by the following equation with the end-group effect on the polymer ignored:
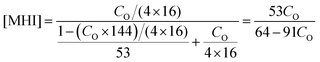 | (5) |
| X (mol/mol) | Conversion (%) | O content in the copolymers (wt%) | Y (mol/mol) |
|---|---|---|---|
| 99/1 | 6.21 | 2.240 | 51.191 |
| 98/2 | 6.43 | 4.336 | 25.132 |
| 97/3 | 6.25 | 6.251 | 16.601 |
| 96/4 | 6.04 | 8.101 | 12.189 |
| 95/5 | 5.98 | 9.812 | 9.590 |
According to the Mayo–Lewis equation, the Fineman–Ross method is expressed as follows:
| G = r1H − r2 | (6) |
G and H are represented by
| G = X(Y − 1)/Y, H = X2/Y | (7) |
| X = [M1]/[M2], Y = d[M1]/d[M2] | (8) |
The plot of G versus H gives a straight line with the slope as r1 and the intercept of the y axis as r2.
The linear relationship equations proposed by Kelen and Tüdõs are as follows:
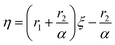 | (9) |
η and ξ are represented by
| η = G/(α + H), ξ = H/(α + H) | (10) |
α is an arbitrary constant and represented by
| α = (HmHM)−1/2 | (11) |
The reactivity ratios of AN and MHI determined by Fineman–Ross and Kelen–Tüdõs methods are compiled in Table 2. Obviously, the reactivity ratios obtained from these two methods show good agreement and the reactivity ratio value of MHI is larger than that of AN, indicating the comonomer MHI possesses higher reactivity than AN. Therefore, the propagating radicals of ∼∼∼AN˙ and ∼∼∼MHI˙ have a preference for comonomer MHI and the comonomer MHI has better chance to incorporate into the polymer chains than AN. It is expected the content of MHI is richer in the P(AN-co-MHI) copolymers than in the feed, the evidence can be found in following element analysis. Comparing with the solution polymerization of AN and MHI in DMSO in our previous work,23 the reactivity ratios of MHI is larger in the mixed solvents polymerization (2.52) than that in solution polymerization of DMSO (2.43) under the same reaction conditions, on the contrary the reactivity of AN is smaller in mixed solvents polymerization (0.52) than in solution polymerization (0.54). The solubility of AN is smaller in DMSO/deionized water = 60/40 (wt/wt) than in DMSO due to the low solubility of AN in water (9 wt% at 60 °C), which reduces the actual concentration of AN in the reaction media of DMSO/deionized water = 60/40 (wt/wt). While the comonomer MHI can dissolve completely in both DMSO and mixed solvents of DMSO/deionized water. For the above reason the reactivity ratios of MHI in the mixed solvents polymerization is larger than in solvent polymerization under the same reaction conditions.
| Method | r1 (AN) | r2 (MHI) |
|---|---|---|
| Fineman–Ross | 0.52 | 2.52 |
| Kelen–Tüdõs | 0.52 | 2.34 |
3.2 Molecular weight and elemental analysis studies
The effect of monomer feed ratios (AN/MHI) on the conversion of polymerization and molecular weight of P(AN-co-MHI) are shown in Fig. 2. Both the conversion of polymerization and molecular weight of P(AN-co-MHI) reduce with the increase of MHI amounts in the feed as shown in Fig. 2. The possible reason is that MHI has larger molecular volume than AN, which counteracts the growth of polymer chains and reduces the polymerization conversion and molecular weight.24 The similar results can be found in our previous work for the solution polymerization of AN with MHI in DMSO. It is worth to point out that the molecular weight of P(AN-co-MHI) prepared in the mixed solvents of DMSO/deionized water = 60/40 (wt/wt) is twice of that prepared in the solvent DMSO under the same conditions because of the small chain transfer constant of deionized water. It is well known that the molecular weight of acrylonitrile copolymers has great effect on the performance of resulting carbon fiber. For the P(AN-co-MHI) copolymer prepared in the mixed solvents of DMSO/deionized water in this work, the molecular weight of P(AN-co-MHI) can meet the requirement of high performance carbon fiber when the monomer feed ratios of AN/MHI is larger than 97/3 (mol/mol). In industry production the polymerization conversion should also be taken into account, the amounts of MHI in the feed should be controlled less than 2.0 mol% based on total monomers in order to get high conversion of polymerization.Elemental analysis of P(AN-co-MHI) copolymers with different molar feed ratios of AN/MHI are shown in Fig. 3. The oxygen element in the copolymers is only provided by the comonomer MHI. As shown in Fig. 3, the O content of P(AN-co-MHI) copolymers increases with the increase of MHI amounts in the feed, while the contents of C, N and H reduce, indicating the increase of MHI content in P(AN-co-MHI) copolymers. In addition, the content of O in the feed (Of) is also shown in Fig. 3. It is found that the content of O is much larger in P(AN-co-MHI) copolymers than in the feed, suggesting the richer content of MHI in P(AN-co-MHI) copolymers, which confirms the above reactivity ratio results.
 | ||
| Fig. 3 Elemental analysis of P(AN-co-MHI) copolymers with different molar feed ratios of AN/MHI: Of, O content in the feed. | ||
3.3 FTIR studies
The FTIR spectra of PAN and P(AN-co-MHI) copolymers with different molar feed ratios of AN/MHI are shown in Fig. 4(a). As shown in Fig. 4(a), the bands at 1731 cm−1 assigned to stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in MHI appears in the FTIR spectra of P(AN-co-MHI) copolymers,28 suggesting the comonomer MHI has copolymerized with AN successfully. Furthermore, the intensity of C
O in MHI appears in the FTIR spectra of P(AN-co-MHI) copolymers,28 suggesting the comonomer MHI has copolymerized with AN successfully. Furthermore, the intensity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption bands becomes stronger with the increase of MHI amounts in the feed, hinting the MHI content in the P(AN-co-MHI) copolymers increases, which is consistent with the above elemental analysis results. While the intensity of bands at 2244 cm−1 assigned to stretching vibration of C
O absorption bands becomes stronger with the increase of MHI amounts in the feed, hinting the MHI content in the P(AN-co-MHI) copolymers increases, which is consistent with the above elemental analysis results. While the intensity of bands at 2244 cm−1 assigned to stretching vibration of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N does not show obvious change in all the polymers, indicating the presence of long uninterrupted sequences of AN units in all acrylonitrile copolymers.29 The intensity of the bands at 2939 cm−1 assigned to the C–H stretching vibration of CH, CH2, and CH3 (ref. 30 and 31) and the bands at 1454 assigned to bending vibration of CH2 keep unchanged in all polymers.
N does not show obvious change in all the polymers, indicating the presence of long uninterrupted sequences of AN units in all acrylonitrile copolymers.29 The intensity of the bands at 2939 cm−1 assigned to the C–H stretching vibration of CH, CH2, and CH3 (ref. 30 and 31) and the bands at 1454 assigned to bending vibration of CH2 keep unchanged in all polymers.
The FTIR spectra of PAN and P(AN-co-MHI) copolymers with different compositions stabilized in air at 200 °C for 30 minutes are shown in Fig 4(b). It is well known that a variety of exothermic chemical reactions, such as cyclization, dehydrogenation and oxidation reactions, occur during the stabilization. The cyclization reactions turn –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups into –C
N groups into –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– structure and the dehydrogenation reactions convert –CH2–CH2– structure into –CH
N– structure and the dehydrogenation reactions convert –CH2–CH2– structure into –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– structure, thus the intensity changes of bands at 2244 cm−1 assigned to stretching vibration of C
CH– structure, thus the intensity changes of bands at 2244 cm−1 assigned to stretching vibration of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and bands at 1629 cm−1 assigned to stretching vibration of C
N and bands at 1629 cm−1 assigned to stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N conjugated with C
N conjugated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C can be used to evaluate the extent of stabilization. In order to evaluate the extent of stabilization, a parameter (Es) is defined as follows:
C can be used to evaluate the extent of stabilization. In order to evaluate the extent of stabilization, a parameter (Es) is defined as follows:
| Es = A1618 cm−1/A2244 cm−1 | (12) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups to the residual C
C groups to the residual C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups.32 The calculated values of Es can be used to represent the extent of stabilization. The molar ratio of MHI/AN in the P(AN-co-MHI) copolymers can be calculated from the elemental analysis of O in Fig. 3, and the plot of Es versus molar ratio of MHI/AN in P(AN-co-MHI) copolymers is shown in Fig. 5.
N groups.32 The calculated values of Es can be used to represent the extent of stabilization. The molar ratio of MHI/AN in the P(AN-co-MHI) copolymers can be calculated from the elemental analysis of O in Fig. 3, and the plot of Es versus molar ratio of MHI/AN in P(AN-co-MHI) copolymers is shown in Fig. 5.
As shown in Fig. 5, the extent of stabilization of P(AN-co-MHI) increases with the increase of MHI content in P(AN-co-MHI) copolymers. The extent of stabilization plays an important role in the performance of resulting carbon fiber and the stabilization reactions should proceed as fully as possible to get ideal performance of carbon fiber.33,34 Based on Fig. 5, it can be concluded that the incorporation of MHI into PAN chains improves the stabilization of PAN effectively, which is beneficial to preparing high performance carbon fiber. It has been widely accepted that the cyclization of nitrile groups in PAN homopolymer can only be initiated through a free radical mechanism. While the cyclization in P(AN-co-MHI) copolymers can be initiated through both a radical mechanism and an ionic mechanism,35 because the hydroxyl oxygen of carboxyl (–COOH) in MHI can make a nucleophilic attack on the carbon atom of adjacent nitrile groups and then induce molecules to cyclize, which can promote the cyclization of P(AN-co-MHI) significantly.
3.4 XRD studies
XRD patterns of PAN and P(AN-co-MHI) copolymers with different compositions are shown in Fig. 6(a). The strongest diffraction peak at about 2θ = 17°, corresponded to a crystalline planar spacing of d = 0.522 nm, is attributed to (100) the crystalline plane of the pseudohexagonal cell.36 The weak diffraction peak at about 2θ = 29° (d = 0.30 nm) is assigned to the (101) crystalline plane of the pseudohexagonal cell.36 XRD patterns of acrylonitrile polymers were processed via Origin 8.0 software to analyze the peak center and the full width at half maximum height (FWHM) around 2θ = 17°. Based on the above data, the corresponding crystalline planar spacing d, crystallite size Lc and crystallinity index CI can be calculated. All the data are tabulated in Table 3. Obviously, the crystallinity index CI of PAN is larger than that of P(AN-co-MHI) copolymers and decreases with increase of MHI content in P(AN-co-MHI) copolymers. The incorporation of MHI into PAN chains blocks the interactions between intermolecular C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups, which reduces the crystallinity of P(AN-co-MHI). While in PAN homopolymer the crystal structure is not destroyed with no MHI incorporated into PAN chains, therefore PAN homopolymer has larger CI. As shown in Fig. 6(a), the peak center around 2θ = 17° hardly moves with the increase of MHI content in P(AN-co-MHI), so the d values of PAN and P(AN-co-MHI) copolymers almost keep unchanged. Additionally, the Lc of P(AN-co-MHI) copolymers is larger than that of PAN homopolymer, which may attribute to the large volume of MHI.
N groups, which reduces the crystallinity of P(AN-co-MHI). While in PAN homopolymer the crystal structure is not destroyed with no MHI incorporated into PAN chains, therefore PAN homopolymer has larger CI. As shown in Fig. 6(a), the peak center around 2θ = 17° hardly moves with the increase of MHI content in P(AN-co-MHI), so the d values of PAN and P(AN-co-MHI) copolymers almost keep unchanged. Additionally, the Lc of P(AN-co-MHI) copolymers is larger than that of PAN homopolymer, which may attribute to the large volume of MHI.
| Molar ratio of MHI/AN | Peak center (o) | FWHW (o) | D (nm) | Lc (nm) | CI (%) |
|---|---|---|---|---|---|
| PAN | 17.16 | 0.93 | 0.522 | 8.840 | 51.04 |
| 0.020 | 17.02 | 0.88 | 0.526 | 9.336 | 43.78 |
| 0.045 | 17.10 | 0.87 | 0.524 | 9.447 | 42.51 |
| 0.070 | 17.04 | 0.83 | 0.526 | 9.899 | 41.78 |
| 0.095 | 17.02 | 0.82 | 0.526 | 10.019 | 41.27 |
| 0.126 | 17.10 | 0.89 | 0.524 | 9.235 | 40.82 |
XRD patterns of PAN and P(AN-co-MHI) copolymers with different compositions heated in air at 200 °C for 30 min are shown in Fig. 6(b). The stabilization is a process transferring linear structure into ladder structure, and the structural changes during the stabilization agree well with the intensity changes of the peak around 2θ = 17°. Thus, the intensity changes around 2θ = 17° can be used to evaluate the extent of stabilization, the equation is expressed as follows:36
| SI = (I0 − Is)/I0 | (13) |
| Molar ratio of MHI/AN | Peak center (o) | Peak intensity I0 (CPS) | Peak intensity Is (CPS) | SI (%) |
|---|---|---|---|---|
| PAN | 17.02 | 5078 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 870 |
−153.45 |
| 0.020 | 16.54 | 3679 | 2891 | 21.42 |
| 0.045 | 16.67 | 3352 | 2554 | 23.81 |
| 0.070 | 16.78 | 2386 | 1324 | 44.51 |
| 0.095 | 16.71 | 1631 | 752 | 53.89 |
| 0.126 | 16.70 | 846 | 382 | 54.85 |
3.5 DSC studies
Fig. 7 show the DSC curves of PAN, P(AN–MA–AA) with molar feed ratio of AN/MA/AA = 98/2/2 and P(AN-co-MHI) copolymers with different compositions heated at 10 °C min−1 from ambient temperature to 375 °C under N2 (40 mL min−1), and the enlarged DSC curves between 180 and 124 °C was inserted in the top left corner of Fig. 7. The parameters obtained from the exotherms, including the temperature of initiation (Ti), the temperature of termination (Tf) and their difference (ΔT = Tf − Ti), the first peak temperature (Tp1, the peak at lower temperature), the second peak temperature (Tp2, the peak at higher temperature), the evolved heat (ΔH), and the velocity of evolving heat (ΔH/ΔT), are listed in Table 5. | ||
| Fig. 7 DSC curves of PAN, P(AN–MA–AA) with molar feed ratios AN/MA/AA = 98/2/2 and P(AN-co-MHI) copolymers with compositions. | ||
| Molar ratio of MHI/AN | Ti (°C) | Tp1 (°C) | Tp2 (°C) | Tf (°C) | ΔT (°C) | ΔH (J g−1) | ΔH/ΔT (J g−1 °C−1) |
|---|---|---|---|---|---|---|---|
| PAN | 244.16 | 278.99 | 303.11 | 58.95 | 2004.67 | 34.01 | |
| P(AN–MA–AA) | 216.89 | 287.46 | 318.05 | 101.16 | 2006.46 | 19.83 | |
| 0.020 | 177.53 | 213.35 | 277.81 | 337.36 | 159.83 | 2146.50 | 13.43 |
| 0.045 | 176.59 | 214.00 | 280.02 | 345.61 | 169.02 | 2227.22 | 13.18 |
| 0.070 | 175.58 | 210.39 | 286.58 | 355.01 | 179.43 | 2312.75 | 12.89 |
| 0.095 | 170.72 | 213.68 | 292.03 | 366.90 | 196.18 | 2490.81 | 12.70 |
| 0.126 | 167.61 | 215.80 | 298.20 | 373.19 | 205.58 | 2513.10 | 12.22 |
The DSC curves were obtained under N2 atmosphere and no oxidative reactions occurred during this process, so the DSC exotherms of acrylonitrile polymers are attributed to the cyclization reactions. As shown in Fig. 7, there is only one exothermic peak in PAN homopolymer and the cyclization reactions can only be initiated through a free radical mechanism. This causes a large amount of heat to be released simultaneously, which leads to the breakage of molecular chains and finally results in structural defects in the resulting carbon fiber. Although the initiation temperature of P(AN–MA–AA) decreases from 244 °C to 216 °C and the ΔH/ΔT of P(AN–MA–AA) reduces from 34.01 J g−1 °C−1 to 19.83 J g−1 °C−1 compared with PAN homopolymer as shown in Table 5, the heat release of P(AN–MA–AA) is still concentrative and expeditious because there is only one exothermic peak in P(AN–MA–AA) as shown in Fig. 7, which is not beneficial to making high performance carbon fiber. Whereas in P(AN-co-MHI) copolymers, there are two exothermic peaks and the cyclization reactions can be initiated through radical and ionic mechanism.35 The lower exothermic peak (peak 1) is assigned to cyclization reactions initiated by MHI through ionic mechanism, which broadens the exothermic peak and avoids centralized heat release. Furthermore, the area of peak 1 increase with the increase of MHI content in P(AN-co-MHI) copolymers as shown in the enlarged DSC curves between 180 and 124 °C on the top left corner of Fig. 7, suggesting that more cyclization reactions are initiated by MHI through ionic mechanism, which promotes the stabilization of P(AN-co-MHI).
As shown in Table 5, the Ti of P(AN-co-MHI) copolymers (ca. 175 °C) is much lower than that of PAN homopolymer (ca. 244 °C) and P(AN–MA–AA) (ca. 216 °C), suggesting a greater ease of initiation of cyclization reactions in P(AN-co-MHI) copolymers. However, the Tf becomes higher with the increase of MHI content in P(AN-co-MHI), indicating the separation of ionic cyclization reactions and radical cyclization reactions. PAN homopolymer has the smallest ΔT and the largest ΔH/ΔT as shown in Table 5, implying that the heat release is concentrative and expeditious. The incorporation of MHI into PAN chains can slows the velocity of evolving heat, as evidenced from the larger ΔT and smaller ΔH/ΔT in the P(AN-co-MHI) copolymers. In addition, both ΔH and ΔT has a tendency to become larger with the increase of MHI content in P(AN-co-MHI) copolymers, while ΔH/ΔT has a tendency to become smaller. Apparently, the synthesized comonomer MHI has a function of relaxing heat release during stabilization, which is beneficial to making high performance carbon fiber.
3.6 Evaluation of activation energy (Ea) of cyclization reactions
Fig. 8 shows DSC curves of PAN homopolymer, P(AN–MA–AA) with molar feed ratios of AN/MA/AA = 98/2/2 and P(AN-co-MHI) copolymer with MHI/AN = 0.070 (mol/mol) heated at different rates (5, 10, 15, 20, 30 °C min−1) from ambient temperature to 375 °C under N2 (40 mL min−1). For all acrylonitrile copolymers, the exotherms wholly shift to higher temperature with the increase of heating rate and the exothermic peaks become stronger as shown in Fig. 8. Kissinger37 and Ozawa's methods38 are mostly used to calculate Ea in literatures, because they just require a series of DSC curves heated at different rates to quantify Ea without any prior knowledge of reaction mechanism. These two methods were also used to calculate Ea of the cyclization reactions in this work. The peak temperature of DSC (Tm) used to evaluate Ea shows a regular increase with the increase of heating rate (φ). | ||
| Fig. 8 DSC curves of PAN (a), P(AN–MA–AA) with molar feed ratio of AN/MA/AA = 98/2/2 (b) and P(AN-co-MHI) with MHI/AN = 0.070 (c) heated at different rates. | ||
The equation used by Kissinger's method are as follows:37
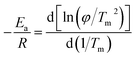 | (14) |
Ea was calculated from the slope of the linear plot of ln(φ/Tm2) versus 1000/Tm as shown in Fig. 9(a).
 | ||
| Fig. 9 Kissinger method (a) and Ozawa method (b) used to quantify Ea: (a) peak 1 of P(AN-co-MHI), (b) peak 2 of P(AN-co-MHI), (c) PAN, (d) P(AN–MA–AA). | ||
Ozawa's method uses the following equation:38
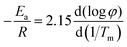 | (15) |
Ea was calculated from the slope of the linear plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) φ versus 1000/Tm as shown in Fig. 9(b).
φ versus 1000/Tm as shown in Fig. 9(b).
The Ea of cyclization reactions of P(AN-co-MHI) copolymers with MHI/AN = 0.020, 0.045, 0.095 and 0.126 (mol/mol) were also calculated by Kissinger and Ozawa's methods and all the results are compiled in Table 6. As shown in Table 6, the Ea determined by Kissinger method agrees well with that obtained by Ozawa method. All DSC curves were obtained under N2 atmosphere, only cyclization reactions occurred during this process. The Ea values of cyclization reactions for PAN homopolymer and P(AN–MA–AA) are about 168 and 128 kJ mol−1, respectively. While in P(AN-co-MHI) the Ea has been splitted into two parts: the first part assigned to the ionic cyclization reactions is calculated from the first exothermic peak and the Ea is about 105 kJ mol−1; the second part calculated from the second exothermic peak is about 200 kJ mol−1, which is assigned to the radical cyclization reactions. Obviously, the Ea of P(AN-co-MHI) has been reduced significantly compared with that of PAN homopolymer and P(AN–MA–AA), which is mainly attribute to the change of cyclization mechanism caused by MHI.24 The decrease of Ea in P(AN-co-MHI) copolymers further confirms that the cyclization of nitrile groups has been promoted by the comonomer MHI, which is beneficial to stabilization of PAN. It is worth to point out that the second part of Ea for P(AN-co-MHI) copolymers is larger than that of PAN homopolymer and P(AN–MA–AA). The possible reason is that once the nitrile groups are initiated by MHI through ionic mechanism the cyclization reactions occurs along the polymer chains, which makes the remaining nitrile groups hard to initiate.
| Molar ratio of MHI/AN | Kissinger (kJ mol−1) | Ozawa (kJ mol−1) | ||
|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 1 | Peak 2 | |
| PAN | 168.39 | 165.89 | ||
| P(AN–MA–AA) | 128.06 | 128.33 | ||
| MHI/AN = 0.020 | 105.67 | 184.22 | 106.32 | 180.64 |
| MHI/AN = 0.045 | 108.05 | 181.34 | 108.53 | 177.93 |
| MHI/AN = 0.070 | 103.22 | 283.82 | 103.95 | 273.76 |
| MHI/AN = 0.095 | 104.04 | 236.63 | 104.85 | 229.81 |
| MHI/AN = 0.126 | 100.38 | 209.64 | 101.39 | 204.71 |
4. Conclusions
The P(AN-co-MHI) copolymers used as carbon fiber precursor were prepared by mixed solvents polymerization of DMSO/deionized water = 60/40 (wt/wt). Both the conversion of polymerization and molecular weight reduce with the increase of MHI amounts in the feed due to the larger molecular volume of MHI. The molecular weight of P(AN-co-MHI) prepared in the mixed solvents of DMSO/deionized water = 60/40 (wt/wt) is twice of that prepared in the solvent DMSO under the same conditions, which can meet the requirement of high performance carbon fiber. The reactivity ratios calculated by Fineman–Ross and Kelen–Tüdõs methods show good agreement and the comonomer MHI possesses higher reactivity than AN, which results in higher content of MHI in P(AN-co-MHI) copolymers than in the feed evidenced by elemental analysis. From the FTIR, DSC and XRD results, it can be found the stabilization of P(AN-co-MHI) copolymers has been significantly improved by MHI with lower initiation temperature, broadened exothermic peak and larger extent of stabilization, which is mainly attributed to the ionic cyclization reactions initiated by MHI. It is further confirmed by the calculation of Ea based on Kissinger method and Ozawa method.Acknowledgements
Financial support of this work from Natural Science Foundation of Jiangsu Province (no. BK20140159), China Postdoctoral Science Foundation (no. 2014M561570), Jiangsu Planned Projects for Postdoctoral Research Funds (no. 1402195C), National Natural Science Foundation of China (no. 21401080) and Important National Research Program “863” (no. 2012AA030313-1) was gratefully acknowledged.Notes and references
- Y. Furushima, M. Nakada, H. Takahashi and K. Ishikiriyama, Study of melting and crystallization behavior of polyacrylonitrile using ultrafast differential scanning calorimetry, Polymer, 2014, 55, 3075 CrossRef CAS PubMed.
- M. G. Huson, J. S. Church, A. A. Kafi, A. L. Woodhead, J. Khoo, M. S. R. N. Kiran, J. E. Bradby and B. L. Fox, Heterogeneity of carbon fibre, Carbon, 2014, 68, 240 CrossRef CAS PubMed.
- Y. Gong, R. Du, G. Mo, X. Q. Xing, C. X. Lü and Z. H. Wu, A in situ microstructural changes of polyacrylonitrile based fibers with stretching deformation, Polymer, 2014, 55, 4270 CrossRef CAS PubMed.
- B. Kumar, M. Asadi, D. Pisasale, S. Sinha-Ray, B. A. Rosen, R. Haasch, J. Abiade, A. L. Yarin and A. S. Khojin, Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction, Nat. Commun., 2013, 4, 2819 Search PubMed.
- J. Zhong, E. K. Kim, J. P. McGann, S. E. Chun, J. F. Whitacre, M. Jaroniec, K. Matyjaszewski and T. Kowalewski, Electrochemically active nitrogen-enriched nanocarbons with welldefined morphology synthesized by pyrolysis of self-assembled block copolymer, J. Am. Chem. Soc., 2012, 134, 14846 CrossRef PubMed.
- J. Zhong, S. Y. Jiang, Y. F. Tang, E. Gottlieb, E. K. Kim, A. Star, K. Matyjaszewski and T. Kowalewski, Lock copolymer-templated nitrogen-enriched nanocarbons with morphology-dependent electrocatalytic activity for oxygen reduction, Chem. Sci., 2014, 5, 3315 RSC.
- W. Mao, F. Simeon, G. C. Rutledge and T. A. Hatton, Electrospun carbon nanofiber webs with controlled density of states for sensor applications, Adv. Mater., 2013, 25, 1309 CrossRef PubMed.
- J. Zhong, S. Natesakhawat, J. P. Baltrus, D. Luebke, H. Nulwala, K. Matyjaszewski and T. Kowalewski, Copolymer-templated nitrogen-enriched porous nanocarbons for CO2 capture, Chem. Commun., 2012, 48, 11516 RSC.
- T. Le, H. Kim, A. Ghosh, J. Kim, J. Chang, Q. A. Vu, D. T. Pham, J. H. Lee, S. W. Kim and Y. H. Lee, Coaxial fiber supercapacitor using all-carbon material electrodes, ACS Nano, 2013, 7, 5940–5947 CrossRef PubMed.
- H. An and H. J. Ahn, Activated porous carbon nanofibers using Sn segregation for high-performance electrochemical capacitors, Carbon, 2013, 65, 87–96 CrossRef PubMed.
- D. Xu, Q. Xu, K. X. Wang, J. Chen and Z. M. Chen, Fabrication of free-standing hierarchical carbon nanofiber/graphene oxide/polyaniline films for supercapacitors, ACS Appl. Mater. Interfaces, 2014, 6(1), 200–209 CAS.
- E. Yang, I. Jang, M. Kim, S. H. Baeck, S. Hwang and S. E. Shim, Electrochemically polymerized vine-like nanostructured polyaniline on activated carbon nanofibers for supercapacitor, Electrochim. Acta, 2013, 111, 136–143 CrossRef PubMed.
- K. Sahin, N. A. Fasanella, I. Chasiotis, K. M. Lyons, B. A. Newcomb, M. G. Kamath, H. G. Chae and S. Kumar, High strength micron size carbon fibers from polyacrylonitrile–carbon nanotube precursors, Carbon, 2014, 77, 442 CrossRef CAS PubMed.
- O. K. Park, S. Lee, H. I. Joh, J. K. Kim, P. H. Kang, J. H. Lee and B. C. Ku, Effect of functional groups of carbon nanotubes on the cyclization mechanism of polyacrylonitrile (PAN), Polymer, 2012, 53, 2168 CrossRef CAS PubMed.
- W. X. Zhang, J. Liu and G. Wu, Evolution of structure and properties of PAN precursors during their conversion to carbon fibers, Carbon, 2003, 41(14), 2805 CrossRef CAS.
- A. Q. Ju, S. Y. Guang and H. Y. Xu, Effect of comonomer structure on the stabilization and spinnability of polyacrylonitrile copolymers, Carbon, 2014, 54, 323 CrossRef PubMed.
- C. L. Lai, G. J. Zhong, Z. R. Yue, G. Chen, L. F. Zhang, A. Vakili, Y. Wang, L. Zhu, J. Liu and H. Fong, Investigation of post-spinning stretching process on morphological, structural, and mechanical properties of electrospun polyacrylonitrile copolymer nanofibers, Polymer, 2011, 52, 519 CrossRef CAS PubMed.
- S.-P. Rwei, T.-F. Way and Y.-S. Hsu, Kinetics of cyclization reaction in poly(acrylonitrile/methyl acrylate/dimethyl itaconate) copolymer determined by a thermal analysis, Polym. Degrad. Stab., 2013, 98, 2072 CrossRef CAS PubMed.
- S. Arbab and A. Zeinolebadi, A procedure for precise determination of thermal stabilization reactions in carbon fiber precursors, Polym. Degrad. Stab., 2013, 98, S CrossRef PubMed.
- Q. Ouyang, L. Cheng, H. J. Wang and K. X. Li, Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile, Polym. Degrad. Stab., 2008, 93, 1415 CrossRef CAS PubMed.
- P. Catta, S. Sakata, G. Garcia, J. P. Zimmermann, F. Galembeck, F. Galembeck and G. Giovedi, Thermal behavior of polyacrylonitrile polymers synthesized under different conditions and comonomer compositions, J. Therm. Anal. Calorim., 2007, 87(3), 657 CrossRef PubMed.
- K. Sen, P. Bajaj and T. V. Speekumar, Thermal behavior of drawn acrylic fibers, J. Polym. Sci., Part B: Polym. Phys., 2003, 41, 2949 CrossRef CAS.
- A. Q. Ju, H. Y. Xu and M. Q. Ge, Preparation and thermal properties of poly[acrylonitrile-co-(β-methylhydrogen itaconate)] used as carbon fiber precursor, J. Therm. Anal. Calorim., 2014, 115, 1037 CrossRef CAS PubMed.
- Y. Q. Zhao, C. G. Wang, Y. X. Wang and B. Zhu, Aqueous deposited copolymerization of acrylonitrile and itaconic acid, J. Appl. Polym. Sci., 2009, 111, 3163 CrossRef CAS.
- V. B. Gupta and S. Kumar, The effect of heat setting on the structure and mechanical properties of poly(ethylene terephthalate) fiber. I. Structural changes, J. Appl. Polym. Sci., 1981, 26, 1865 CrossRef CAS.
- M. Fineman and S. D. Ross, Linear method for determining monomer reactivity ratios in copolymerization, J. Polym. Sci., 1950, 5, 259 CrossRef CAS.
- T. Kelen and F. Tüdõs, Analysis of the linear methods for determining copolymerization reactivity ratios. I. New improved linear graphic method, J. Macromol. Sci., Chem., 1975, 9, 1 CrossRef.
- N. U. Nguyen-Thai and S. C. Hong, Structural evolution of poly(acrylonitrile-co-itaconic acid) during thermal oxidative stabilization for carbon materials, Macromolecules, 2013, 46, 5882 CrossRef CAS.
- O. P. Bahl, R. B. Mathur and N. K. Sandle, IR studies of PAN fibers thermally stabilized at elevated temperatures, Carbon, 1994, 32, 1133 CrossRef.
- S. W. Lee, H. Y. Lee, S. Y. Jang, S. Jo, H. Lee, W. H. Choe, S. Lee and J. Mittal, Efficient preparation of carbon fibers using plasma assisted stabilization, Carbon, 2013, 55, 361 CrossRef CAS PubMed.
- T. Usami, T. Itoh, H. Ohtani and S. Tsuge, Structural study of polyacrylonitrile fibers during oxidative thermal degradation by pyrolysis-gas chromatography, solid-state carbon-13 NMR, and Fourier-transform infrared spectroscopy, Macromolecules, 1990, 23, 2460 CrossRef CAS.
- Q. Ouyang, L. Cheng, H. J. Wang and K. X. Li, Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile, Polym. Degrad. Stab., 2008, 93, 1415 CrossRef CAS PubMed.
- W. Watt, Carbon work at the royal aircraft establishment, Carbon, 1972, 10, 121 CrossRef CAS.
- O. P. Bahl and L. M. Manocha, Characterization of oxidised PAN fibres, Carbon, 1974, 12, 417 CrossRef CAS.
- A. Q. Ju, S. Y. Guang and H. Y. Xu, Mechanism and kinetics of stabilization reactions of poly(acrylonitrile-co-β-methylhydrogen itaconate), J. Mater. Res., 2012, 27(20), 2668 CrossRef CAS.
- M. J. Yu, Y. J. Bai, C. G. Wang, Y. Xu and P. A. Guo, A new method for the evaluation of stabilization index of polyacrylonitrile fibers, Mater. Lett., 2007, 61, 2292 CrossRef CAS PubMed.
- H. E. Kissinger, Reaction kinetics in differential thermal analysis, Anal. Chem., 1957, 29, 1702 CrossRef CAS.
- T. Ozawa, A new method of analyzing thermogravimetric data, Bull. Chem. Soc. Jpn., 1965, 38, 1881 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |





