Topotactic synthesis and photocatalytic performance of one-dimensional ZnNb2O6 nanostructures and one-dimensional ZnNb2O6/KNbO3 hetero-nanostructures
Abstract
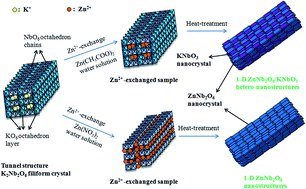
This paper introduces one-dimensional ZnNb2O6/KNbO3 hetero-nanostructures and one-dimensional ZnNb2O6 nanostructures. These nanostructures are synthesized via in situ topotactic structural transformation reaction using the tunnel structure K2Nb2O6 filiform crystal as precursor. Firstly, Zn2+ ions intercalate into K2Nb2O6 crystal by exchanging K+ ions from the K2Nb2O6 crystal with Zn2+ from Zn(NO3)2 or Zn(CH3COO)2 aqueous solution, to form two different Zn2+-exchanged samples, and then these Zn2+-exchanged samples topotacticly transform into one-dimensional ZnNb2O6/KNbO3 hetero-nanostructures and ZnNb2O6 nanostructures during heat-treatment. The formation reaction and structure of these samples were characterized by X-ray diffraction (XRD), field-emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), selected-area electron diffraction (SAED), and energy-dispersive spectroscopy (EDS). Photocatalytic experiments showed that one-dimensional ZnNb2O6/KNbO3 hetero-nanostructures and ZnNb2O6 nanostructures have excellent photocatalytic performance for the degradation of methylene blue (MB), rhodamine B (RhB), and methyl orange (MO).

 Please wait while we load your content...
Please wait while we load your content...