Novel zwitterionic deep eutectic solvents from trimethylglycine and carboxylic acids: characterization of their properties and their toxicity†
Abstract
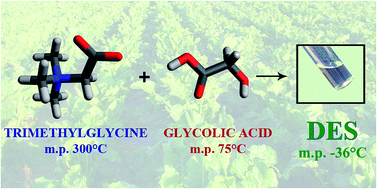
We report the preparation and the study of the properties of novel deep eutectic solvents (DESs) formed by zwitterionic trimethylglicine and high melting point carboxylic acids. These zwitterionic DESs are liquid at room temperature or at temperatures lower than 70 °C and do not have chloride or any metal ions in their composition. These media were characterized in terms of their viscosity, conductivity, density, ionicity (via Walden plots), surface tension and thermal stability. The values observed are similar to other typical DESs, and the mixtures resulted in “poor ionic liquids” in the Walden plot. The values obtained could be correlated to the carboxylic acids' structures. The toxicity of the pure mixtures was evaluated via an FTIR-bioassay on Saccharomyces cerevisiae cells. This method allowed us to define the action of these media as dehydrating agents on eukaryotic model cells, with a mechanism highly correlated with CaCl2, a well-known dehydrating agent. The Glycolic acid/Trimethylglicine eutectic system can be considered a NADES (Natural Deep Eutectic Solvent) and resulted as the best in our set for many reasons. It is formed by natural, renewable, eco-compatible and cheap compounds (both the molecules can be derived from sugar beet) and it can be used in many applications because it has a low melting point (−36 °C) and it is colorless, and therefore suitable for spectroscopic measurements. For these reasons, the solubility of some α-L-amino acids was investigated in this DES, showing a good solubility for aromatic amino acids, which are normally scarcely soluble in water.

 Please wait while we load your content...
Please wait while we load your content...