Efficient synthesis of pyrene-1-carbothioamides and carboxamides. Tunable solid-state fluorescence of pyrene-1-carboxamides†
Abstract
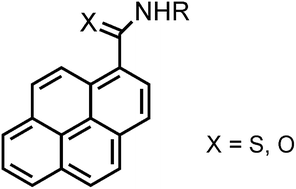
Pyrene reacts with potassium thiocyanate and organic isothiocyanates in the presence of trifluoromethanesulfonic acid to afford primary and secondary pyrene-1-carbothioamides in high yields. These compounds were efficiently oxidatively desulfurized with Oxone® to the corresponding carboxamides. The amides display solid-state fluorescence with quantum efficiencies up to 62%, originating from monomers, aggregates (such as preformed dimers), and/or excimers, depending on the substituent at the nitrogen atom. Single crystal X-ray diffraction characterization of one highly emissive compound supports this assumption.

 Please wait while we load your content...
Please wait while we load your content...