β-Sitosterol-D-glucopyranoside isolated from Desmostachya bipinnata mediates photoinduced rapid green synthesis of silver nanoparticles†
Khan Behlol Ayaz Ahmed‡
a,
Shankar Subramaniam‡a,
Ganapathy Veerappanb,
Natarajan Haria,
Aravind Sivasubramanian *a and
Anbazhagan Veerappan*a
*a and
Anbazhagan Veerappan*a
aDepartment of Chemistry, School of Chemical and Biotechnology, SASTRA University, Thanjavur – 613 401, Tamil Nadu, India. E-mail: anbazhagan@scbt.sastra.edu.in; arvi@biotech.sastra.edu; Fax: +91-4362-26120; Tel: +91-4362-264101-3689
bSKKU Advanced Institute of Nano Technology, Sungkyunkwan University, Suwon, South Korea
First published on 30th October 2014
Abstract
In this work, we report a completely green chemistry approach to synthesize silver nanoparticles (AgNPs) using the compound β-sitosterol-D-glucopyranoside (BS) isolated from the Indian sacred grass Desmostachya bipinnata, using natural sunlight as the initiator. BS synthesizes the AgNPs, in a free radical exchange process involving reactive oxygen species. The possible mechanism of the influence of BS on the reduction and stabilization of AgNPs is also discussed. The optical properties and morphology of the AgNPs were characterized by absorption spectroscopy and transmission electron microscopy. These bio-inspired AgNPs exhibited good catalytic activity during the catalytic degradation of environmentally hazardous organic dyes like methylene blue, methyl red, Congo red and acridine orange, in aqueous media.
1. Introduction
Silver nanoparticles (AgNPs) are of great interest and importance for applications in optics, electronics, catalysis, sensors, surface-enhanced Raman scattering and health care.1 Due to the wide range of applications of AgNPs, various methods have been developed to synthesise metal nanoparticles. Among these methods, the most preferred synthetic route is the wet chemical reduction of metals salts in their liquid phase in the presence of a stabilizer and reducing agents.2 The negative impacts caused by hazardous chemicals used in the wet chemical reduction method lead to the development of green nanotechnologies that employ naturally occurring materials, such as plant extracts, bacteria, fungus and bio-derived chemicals, in synthesizing stable nanomaterials.3 Among other bio-inspired processes for synthesizing NPs, plant extract mediated syntheses have been considered more advantageous over other environmentally benign biological processes, because it eliminates the elaborate process of maintenance.4 Moreover, it is eco-friendly, cost-effective and very easy to scale up for large-scale production. Despite its advantages, the use of plant extract suffers from uncertainty about the chemical components responsible for the synthesis and function of the nanoparticles. Because of this, it is difficult to predict the formation mechanism of nanoparticles using plant extract. However, there are studies that report the involvement of polyphenols and flavonoids in the synthesis of AgNPs.4 To get more detailed insight into plant extract mediated nanoparticles syntheses, it is imperative to identify the exact phytoconstituents responsible for nanoparticle formation.An essential criterion for any green nanotechnology is an environmentally non-hazardous solvent system and synthetic producer. Solar energy is believed to be the largest source of carbon neutral renewable energy. Moreover, sunlight is non-toxic and non-polluting.5 Recently, Zarchi and co-workers reported the sunlight induced synthesis of silver nanoparticles using an ethanol extract of Andrachnea chordifolia. It was shown that the toxicity of AgNPs was considerably reduced when it is prepared by sunlight.6 Gold nanostructures prepared using sunlight irradiation have been reported as a selective sensor for lead ions.7 Sunlight induced synthesis of silver nanoparticles on Citrus limetta aqueous extract has been reported as a selective sensor for mercury ions.8 Bimetallic silver–gold nanostructures synthesized via sunlight irradiation on a DNA template found application in TNT/tumor maker detection.9
Herein, we report the sunlight mediated synthesis of silver nanoparticles using the aqueous extract of Desmostachya bipinnata. D. bipinnata is one of the sacred grasses used in Indian vedic rituals and ceremonies and its medicinal properties have been well reported.10 It is also used as a fodder crop in semi-arid regions.11 We have isolated a sterol glycoside, β-sitosterol-D-glucopyranoside (BS) from its aqueous extract. This particular compound, BS, has been shown to reduce Ag+ to Ag0 under the influence of sunlight, indicating that the BS contained in the aqueous extract is responsible for the formation of AgNPs. The possible mechanism of the influence of BS on the reduction and stabilization of AgNPs is also discussed. The possible application of AgNPs obtained with D. bipinnata aqueous extract in catalytic degradation of various organic dyes was explored, in aqueous medium.
2. Materials and methods
2.1. Collection and processing of plant
The plants Desmostachya bipinnata were obtained along the river beds of River Cauvery, in Thanjavur District, Tamil Nadu, India. The plant was authenticated by Dr Jayendran, Department of Botany, Government Arts College, Ootacamund, India. All voucher specimens after matching with the authenticated herbarium sample in the RAPINAT Herbarium, Trichy, India, were deposited in Government Arts College, Ootacamund, India.2.2. Preparation of plant aqueous extract
The shade dried leaves and aerial parts were ground to fine powder and used for extraction. The aqueous extract was prepared by soaking 1 kg of plant material in Millipore water at 30 °C for 24 h and filtered through Whatman no. 1 filter paper. The extraction was done three times and pooled up, and then was concentrated under vacuum with a rotary evaporator (Buchi® Rotavap R-210).2.3. Typical synthesis of silver nanoparticles from the plant aqueous extract
In a typical procedure, 50 mg of lyophilized aqueous extract of D. bipinnata was mixed with 50 mL of deionized water and filtered through Whatman filter paper no. 41. To this extract, 1 mM AgNO3 aqueous solution is added with stirring at room temperature. D. bipinnata extract produces colour change with AgNO3 solution after keeping for 48 h under room light. However, upon exposure to sunlight, the colourless silver nitrate solution was converted into a pale yellow colour within a minute and drastically deepened to a yellow colour in 5 min. AgNPs can also be prepared by irradiating the D. bipinnata extract with 1 mM AgNO3 under UV-light.2.4. Isolation of β-sitosterol-D-glucopyranoside (BS)
The aqueous extract of D. bipinnata (30 g) was subjected to column chromatography with silica gel (60–120 mesh) packed in a glass column of 4 × 45 cm with bed height of 30 cm and elution with chloroform with increasing concentration of MeOH. The column elution was monitored by TLC and fractions were pooled based on similar TLC profiles. Fraction 6 and 7 were combined together and subjected to column chromatography with silica gel (230–400 mesh) and eluted with chloroform, chloroform–methanol (2, 4, 8, 10 and 20%) and methanol successively. The compound, BS was obtained from this column chromatography. The structure of the compound was confirmed by various spectroscopic techniques.2.5. Characterization
UV-vis spectra of the silver nanoparticle solutions were recorded on a Thermo Scientific Evolution 201 spectrophotometer operated at a resolution of 1 nm. The size, morphology and crystallinity of AgNPs were also characterized using high-resolution transmission electron microscopy (HR-TEM) with an accelerated voltage of 200 kV. Samples of TEM measurements were prepared by placing a drop of NP solution on the graphite grid and drying it in vacuum. The concentration of the formed AgNPs was determined by atomic absorption spectroscopy. About 98 μg mL−1 of colloidal silver was present in the solution, which corresponds to 0.92 mM.2.6. Reactive oxygen species and free radical assays
The presence of reactive oxygen species (ROS) in the solution was measured by dichlorofluorescein diacetate (DCFH-DA). Briefly, 1 mg mL−1 of D. bipinnata aqueous extract or BS was incubated with DCFH-DA and exposed to room light and sunlight. DCFH-DA dissolved in water is used as a control. After 10 min, fluorescence was measured on a Jasco FP-8200 spectrofluorimeter operating at a slit width of 2.5 nm. The excitation and emission wavelengths were set at 485 and 528 nm, respectively.The generation of free radicals by D. bipinnata aqueous extract after exposure to sunlight was measured by diphenyl picrylhydrazyl (DPPH) assay. Briefly, 10 μL of 0.1 mM DPPH (dissolved in ethanol) was added to different concentrations of D. bipinnata aqueous extract and exposed to room light and sunlight for 10 min. The reduction of the DPPH free radical was measured by reading the absorbance at 517 nm. Ascorbic acid was used as the positive control. The lower absorbance of the reaction mixture indicated a higher percentage of scavenging activity. The inhibition ratio was calculated from the following equation:
| % scavenging = [(control absorbance − sample absorbance)/(control absorbance)] × 100 |
2.7. Catalytic activity
Dye degradation activities of AgNPs were investigated using the discoloration of methylene blue (MB). Briefly, 15 μM of MB solution were mixed with NaBH4 (50 mM) and 50 μL of AgNPs (1 mM) and the absorption spectra were recorded immediately. The change in absorption at 664 nm was used for kinetic analysis.Since the concentration of NaBH4 was much higher than that of the organic dyes, the degradation kinetics can be described by a first-order rate law.12 Therefore, the reaction kinetics can be described as ln(Ct/C0) = −kt, where k is the apparent first-order rate constant, t is the reaction time. Ct and C0 are the concentrations of substrate at time t and 0, respectively. The rate constant, k was obtained directly from the slope of the linear part of the kinetic trace.
3. Results and discussion
3.1. Silver nanoparticles preparation and characterization
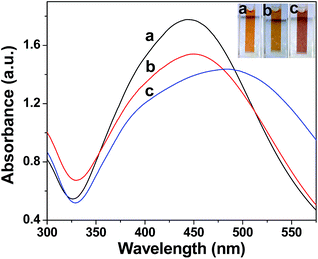
Biogenic AgNPs are usually obtained by incubating AgNO3 solution with aqueous plant extracts.4 Addition of a 1 mM AgNO3 solution into D. bipinnata aqueous extract produced a colour change from colourless to yellow (Fig. 1) after 48 h of incubation and showed a surface plasmon resonance (SPR) absorption peak at 450 nm, characteristics of AgNPs.13 Irradiating the mixture of D. bipinnata aqueous extract and 1 mM AgNO3 with sunlight produces an intense yellow colour within 5 min and shows an SPR absorption peak at 445 nm. This indicates the formation of nanosized silver. In order to confirm the complete reduction of Ag+ to Ag0, sodium borohydride was added to the formed AgNPs. No changes were observed in the UV-vis spectrum, confirming the complete reduction process. Both, room light and sunlight irradiated preparations showed a broad SPR band, which is indicative of polydispersed NPs formation in the aqueous solution.14Since sunlight is also a source of UV radiation, we performed experiments at constant concentration of AgNO3 and D. bipinnata aqueous extract under ambient conditions in the lab using UV light. The exposure to UV light for 3 h produces a colour change from colourless to a brown colour and showed a very broad SPR peak at 485 nm. The position, width of the absorption peak and intensity of the peak depends on the particle size, shape and dielectric constant of the surrounding medium.15 All these experiments clearly indicate the involvement of light in the reduction of Ag+ to Ag0. These results also suggest that the phytomolecules present in the extract are responsible for the photochemical reduction of Ag+ ions.
The dispersity of AgNPs prepared by room light, sunlight and UV-light was evaluated by comparing the full width at half-maximum (FWHM) from the UV-vis spectra.16 The FWHM calculated for AgNPs synthesized at sunlight, room light and UV light are 146 nm, 155 nm and 183 nm, respectively. These analyses suggest that AgNPs synthesized by different light sources are highly polydispersed. The stability of the synthesized AgNPs was determined by measuring their intensities at the peak maximum over a period of 7 days and no significant changes in the intensity were observed.
As inferred from TEM images (Fig. 2), the formed AgNPs are highly polydispersed with diverse shapes, supporting the absorption spectral characteristics. The size of the particles measured from TEM images for the AgNPs prepared at sunlight, room light and UV light are in the range of 10–35 nm, 8–40 nm and 15–70 nm, respectively. About 4 nm thickness of the capping agent stabilizing the NPs was detectable in Fig. 2D, indicating that the phytoconstituents present in D. bipinnata protect the AgNPs from agglomeration. FTIR analysis further confirmed the presence of phytoconstituents around the nanoparticles (Fig. S1†). The observed fringe pattern in HR-TEM (Fig. 2E) and selected area electron diffraction (SAED) pattern supports the crystalline nature of the as-prepared AgNPs (Fig. 2F).
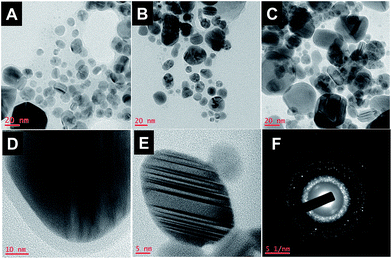 | ||
| Fig. 2 TEM images of AgNPs prepared from aqueous extract of D. bipinnata mediated by (A) sunlight, (B) room light, (C) UV light; (D) & (E) HRTEM and (F) SAED pattern. | ||
3.2. Phytochemical analysis and isolation of BS
In order to identify the compound responsible for the photochemical reduction, we performed column chromatography on the D. bipinnata aqueous extract. The fractions which converted the AgNO3 into AgNPs under sunlight were carefully collected and subjected to further chromatography steps. A compound (BS) was isolated as a white crystalline solid which had a molecular formula of C35H60O6. It responded positively to the Liebermann–Burchard test for steroids.17 Its IR absorption spectrum showed peaks at 3340 cm−1 corresponding to hydroxyl (–OH) stretching, at 1720 cm−1 (corresponding to![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) and absorption bands at 2900–2850 cm−1 indicating the presence of possible methylene and methine groups. The EIMS spectrum had a molecular ion at m/z 414 [M − sugar]+ corresponding to the aglycone-steroid.18
O stretching) and absorption bands at 2900–2850 cm−1 indicating the presence of possible methylene and methine groups. The EIMS spectrum had a molecular ion at m/z 414 [M − sugar]+ corresponding to the aglycone-steroid.18
The 1H NMR spectrum (Fig. 3) showed the presence of an olefinic signal (δH 5.08), indicating a ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond splayed right]](https://www.rsc.org/images/entities/char_e00a.gif) system in the ring. A one proton broad multiplet at δH 4.44 showed a cross peak with C2 protons and the C4 proton in HETCOR and this signal was assigned to the C3 methine proton. A plethora of multiplets was found in the range δH 1.1–2.14 and these were informative about the presence of different methylene and methine protons of the steroidal structure. The other proton resonances were allocated to the glucopyranoside. Further evidence was also provided by the 13C-NMR that showed resonances for 35 carbon atoms. The C3 carbon resonated at 71.73 ppm. The anomeric and the oxygenated methylene carbons of the sugar appeared at 100 and 61 ppm respectively (Fig. S4†). Thus on the basis of the spectral data, the structure of the compound was elucidated as stigmast-5-en-3-O-β-D-glucopyranoside (β-sitosterol glucoside). All the spectral data were in complete concurrence with the literature.19
system in the ring. A one proton broad multiplet at δH 4.44 showed a cross peak with C2 protons and the C4 proton in HETCOR and this signal was assigned to the C3 methine proton. A plethora of multiplets was found in the range δH 1.1–2.14 and these were informative about the presence of different methylene and methine protons of the steroidal structure. The other proton resonances were allocated to the glucopyranoside. Further evidence was also provided by the 13C-NMR that showed resonances for 35 carbon atoms. The C3 carbon resonated at 71.73 ppm. The anomeric and the oxygenated methylene carbons of the sugar appeared at 100 and 61 ppm respectively (Fig. S4†). Thus on the basis of the spectral data, the structure of the compound was elucidated as stigmast-5-en-3-O-β-D-glucopyranoside (β-sitosterol glucoside). All the spectral data were in complete concurrence with the literature.19
3.3. Role of BS in the synthesis of AgNPs
The fraction containing the compound BS produced the AgNPs and exhibited a significant SPR and so did the isolated compound, BS. At constant concentration of AgNO3 and BS under ambient conditions in the lab, sunlight and UV light exposure produces AgNPs with characteristic SPR peaks at 430, 460 and 455 nm, respectively (Fig. 4). It was observed that the sunlight mediated formation of AgNPs occurred in 5 min, whereas the room light and UV light irradiation took 48 h and 3 h, respectively to produce AgNPs. The FWHM calculated for AgNPs synthesized by BS in sunlight, room light and UV light are 137 nm, 168 nm and 174 nm, respectively. Similar observations were made with the aqueous extract of D. bipinnata (Fig. 1). This study confirms that BS present in the aqueous extract is most likely involved in the formation of AgNPs. To further substantiate the involvement of BS in the formation of AgNPs, we isolated BS from other sacred grasses, Imperata cylindrica and Cynodon dactylon, and applied it to AgNPs synthesis. Interestingly, BS isolated from I. cylindrica and C. dactylon also produced AgNPs (unpublished observation).The microscopic structure of the BS mediated silver nanoparticles was investigated by HR-TEM (Fig. S2†). TEM images confirm the formation of silver nanoparticles with the size range between 8 and 60 nm. The SAED pattern supports the formation of polycrystalline silver nanoparticles (Fig. S2†). The size-distribution of nanoparticles was further determined using a particle-size analyzer. The average particle sizes for BS and D. bipinnata aqueous extract stabilized AgNPs were found to be 149 and 183 nm, respectively (Fig. S5†). These values are higher than the values obtained from HRTEM. The difference in size estimated is due to the differences in the techniques. The particle size analyzer measures the size of the cluster rather than the individual particles. However, the observed higher values can also attributed to the presence of phytoconstituents around the AgNPs. Zeta potentials estimated for BS and D. bipinnata aqueous extract stabilized AgNPs were found to be −15.9 and −12.5 mV.16 These values indicate that the AgNPs are stable due to the electrostatic repulsion (Fig. S6†).
3.4. Possible mechanism of AgNPs formation
From the above data, it is clear that a photochemical reaction is involved in the reduction of Ag+ to Ag0. The major requirement of any photochemical electron transfer reaction is the generation of highly reactive species by light. To confirm the production of reactive species (if any) by light, we embarked on a fluorimetric detection of it. The fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) is widely used as an oxidant-sensitizer and is non-fluorescent, and switches to a highly fluorescent DCF, when oxidized by ROS and other peroxides.20 The addition of DCFH-DA to the D. bipinnata extract and BS showed the highest fluorescence intensity in the sunlight irradiated samples compared to samples kept at room light, plausibly due to a higher generation of ROS after sunlight irradiation (Fig. 5A and B). Next, we examined the presence of free radicals using the known free radical scavenger DPPH.21 The samples irradiated with sunlight showed the highest free radical scavenging activity, consistent with the generation of ROS (Fig. 5C). BS alone also exhibited the same profiles with DCFH-DA and DPPH. | ||
| Fig. 5 Reactive oxygen species assay, (A) D. bipinnata aqueous extract and (B) β-sitosterol glucoside. (C) Free radical scavenging assay. | ||
Based on this result, we hypothesized the involvement of a redox reaction in the formation of nanoparticles. Phytosterols are susceptible to photoxidation22 with light through the formation of a transient endoperoxide. While forming the transient endoperoxide, BS releases the electrons for the formation of AgNPs (Scheme 1). This endoperoxide is presumably present in equilibrium under the light. To support the proposed mechanism, we investigated the structure of isolated BS after exposure to sunlight as well as UV light. 1H NMR and 13C NMR clearly showed unaltered structure (Fig. S7 and S8†), indicating that the BS is only mediating the sunlight induced reduction of silver ions to silver nanoparticles by undergoing a transient transformation. However, the amphiphilic property of BS might play an important role in preventing the nanoparticles agglomeration.
3.5. Application of DpAgNPs (D. bipinnata AgNPs) in dye degradation
Organic dye releases from industries are severely affecting the environment by contaminating the soil and water bodies.23 The removal of these pollutants requires expensive procedures, which are not affordable by the small scale industries.24 Of late there is considerable interest in employing noble metal nanoparticles in degrading the organic dyes.25 Recently, green synthesized AgNPs from plant extracts have been shown to degrade methyl red dye under UV light.26 Nevertheless, the sunlight induced synthesized AgNPs from plant extracts that offer an eco-friendly system have never been explored in detail for degrading toxic organic dyes. Methylene blue (MB) has been widely used as a model dye to investigate the degradation of basic dyes.27 MB is widely used in the textile industry for dyeing cotton, wool, silk and acrylic fibers.28 The previous reports on the degradation of MB have been based on hydrogen peroxide, ascorbic acid, photodecomposition on TiO2, photocatalytic degradation on Ag+ doped TiO2,27 while the present study reports the catalytic degradation of MB using biogenic AgNPs and NaBH4.In aqueous medium MB shows an absorption maximum at 664 nm accompanied with a shoulder at 614 nm. Addition of D. bipinnata AgNPs and reducing agent NaBH4 to the MB solution gradually diminishes the blue colour (Fig. 6A). As shown in Fig. 6A, the MB absorption peak decreases in a time dependent manner. In the absence of D. bipinnata AgNPs or in the presence of NaBH4, the peak at 664 nm remains undisturbed for more than 10 min. The discolorization and complete loss of MB absorption peak signify the importance of D. bipinnata AgNPs in degrading the organic dye, methylene blue. In order to follow the rate of degradation, the time dependent change in the intensity at 664 nm was monitored using a UV-vis spectrophotometer (Fig. S9†). The linear correlation between ln(At/A0) and the reaction time indicates the degradation is a pseudo first order reaction. The rate constant is calculated from its slope and is found to be 0.05 s−1 for the degradation of MB assisted by the D. bipinnata AgNPs. It is noteworthy that BS-AgNPs also exhibit a similar effect in degrading MB. This study clearly indicates that the bio-inspired AgNPs reported in this study have the potential to degrade hazardous organic dye releases from industry.
As a proof of concept, we further evaluated the D. bipinnata AgNPs mediated degradation of other organic dyes, methyl red, Congo red and acridine orange (Fig. 6). Irrespective of the dye nature, addition of D. bipinnata AgNPs and NaBH4 gradually diminishes the original colour of the dye to colourless. The discoloration happens in a time dependent manner. To obtain the rate of degradation, we monitored the change in the intensity with respect to time using a UV-vis spectrophotometer (Fig. S9†).
The rate constants for the dye degradation were calculated as detailed in the material and methods section. It is noteworthy that the NaBH4 alone can decolorize the tested organic dyes, but it requires 5 h, 7 h, 8 h and 12 h to decolorize methylene blue, methyl red, Congo red and acridine orange, respectively. Nevertheless, the kinetic analysis reported in Table 1 clearly indicates that the presence of D. bipinnata AgNPs accelerates the rate of degradation and almost all the tested dyes are degraded in less than 2 min. These results clearly indicate the catalytic role of D. bipinnata AgNPs in degrading the organic dyes and a significant improvement in degrading the organic dyes in a shorter time interval.
| Dye | Rate constant (s−1) |
|---|---|
| Methylene blue | 0.057 ± 0.007 |
| Methyl red | 0.038 ± 0.004 |
| Congo red | 0.030 ± 0.003 |
| Acridine orange | 0.025 ± 0.006 |
4. Conclusion
D. bipinnata has been considered as a sacred plant in Indian mythology. For the first time, we reported the preparation of AgNPs using the aqueous extract of D. bipinnata under sunlight irradiation. The use of a natural light source, one-pot process, less reaction time, cost-effectiveness, longer stability and reproducibility, renders this method more advantageous over the other available methods. The identification of bio-surfactant—glycosides—in the aqueous extract further delineated the role of surfactant-like phytomolecules in the formation of AgNPs. The as-prepared D. bipinnata AgNPs exhibited a good catalytic activity during the catalytic degradation of environmentally hazardous organic dyes, methylene blue, methyl red, Congo red and acridine orange, in aqueous solution. Further testing their dye degrading ability on a greater number of hazardous dyes and industrial dye effluent would help in recommending these AgNPs for industrial use.Author contributions
KBAA and SS performed research and analyzed data. AS designed research and characterized D. bipinnata and isolated BS, analyzed data, wrote the manuscript. AV designed research and characterized AgNPs, analyzed data, wrote the manuscript.Acknowledgements
KBAA and SS earnestly acknowledge the teaching assistantship from SASTRA University. AV acknowledges the financial support from Department of Science and Technology, Government of India (SB/FT/LS-217/2012). AS earnestly acknowledges the funding source from Department of Science and Technology, Government of India (SR/FT/CS-10/2011) and the TRR fund.References
- (a) C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS PubMed; (b) R. A. Sperling, P. Rivera Gil, F. Zhang, M. Zanella and W. J. Parak, Chem. Soc. Rev., 2008, 37, 1896 RSC; (c) B. C. Ranu, K. Chattopadhyay, L. Adak, A. Saha, S. Bhadra, R. Dey and D. Saha, Pure Appl. Chem., 2009, 81, 2337 CrossRef CAS; (d) V. Anbazhagan, K. B. A. Ahmed and S. Janani, Sens. Actuators, B, 2014, 200, 92 CrossRef CAS PubMed.
- M. A. Faramarzi and A. Sadighi, Adv. Colloid Interface Sci., 2013, 189–190, 1 CrossRef CAS PubMed.
- (a) N. I. Hulkoti and T. C. Taranath, Colloids Surf., B, 2014, 121C, 474 CrossRef PubMed; (b) K. B. A. Ahmed, D. Kalla, K. B. Uppuluri and V. Anbazhagan, Carbohydr. Polym., 2014, 112, 539 CrossRef CAS PubMed.
- (a) O. V. Kharissova, H. V. Dias, B. I. Kharisov, B. O. Pérez and V. M. Pérez, Trends Biotechnol., 2013, 31, 240 CrossRef CAS PubMed; (b) A. K. Mittal, Y. Chisti and U. C. Banerjee, Biotechnol. Adv., 2013, 31, 346 CrossRef CAS PubMed; (c) K. B. Ayaz Ahmed, S. Subramanian, A. Sivasubramanian, G. Veerappan and A. Veerappan, Spectrochim. Acta, Part A, 2014, 130, 54 CrossRef CAS PubMed; (d) J. Seralathan, P. Stevenson, S. Subramaniam, R. Raghavan, B. Pemaiah, A. Sivasubramanian and A. Veerappan, Spectrochim. Acta, Part A, 2014, 118, 349 CrossRef CAS PubMed; (e) S. Mukherjee, D. Chowdhury, R. Kotcherlakota, S. Patra, B. Vinothkumar, M. P. Bhadra, B. Sreedhar and C. R. Patra, Theranostics, 2014, 4, 316 CrossRef CAS PubMed; (f) S. Li, Y. Shen, A. Xie, X. Yu, L. Qiu, L. Zhang and Q. Zhang, Green Chem., 2007, 9, 852 RSC.
- (a) Y. L. Luo, Mater. Lett., 2008, 62, 3770 CrossRef CAS PubMed; (b) M. Sun, W. C. Hao, C. Z. Wang and T. M. Wang, Chem. Phys. Lett., 2007, 443, 342 CrossRef CAS PubMed.
- A. A. K. Zarchi, N. Mokhtari, M. Arfan, T. Rehman, M. Ali, M. Amini, R. F. Majidi and A. R. Shahverdi, Appl. Phys. A: Mater. Sci. Process., 2011, 103, 349 CrossRef CAS.
- Y. Chien, C. Huang, S. Wang and C. Yeh, Green Chem., 2011, 13, 1162 RSC.
- S. S. Ravi, L. R. Christena, N. SaiSubramanian and S. P. Anthony, Analyst, 2013, 138, 4370 RSC.
- L. Yang, G. Chen, J. Wang, T. Wang, M. Li and J. Liu, J. Mater. Chem., 2009, 19, 6849 RSC.
- S. Subramaniam, M. Keerthiraja and A. Sivasubramanian, Rev. Bras. Farmacogn., 2014, 24, 44–50 CrossRef CAS PubMed.
- S. Gulzar, M. A. Khan and X. Liu, Rangeland Ecol. Manage., 2007, 60, 401–407 Search PubMed.
- P. Herves, M. Perez-Lorenzo, L. M. Liz-Marzan, J. Dzubiella, Y. Lu and M. Ballauff, Chem. Soc. Rev., 2012, 41, 5577 RSC.
- C. Dipankar and S. Murugan, Colloids Surf., B, 2012, 98, 112 CrossRef CAS PubMed.
- R. W. Raut, V. D. Mendhulkar and S. B. Kashid, J. Photochem. Photobiol., B, 2014, 132, 45 CrossRef CAS PubMed.
- E. Kelly, L. Coronado, L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668 CrossRef.
- (a) S. Janani, P. Stevenson and A. Veerappan, Colloids Surf., B, 2014, 117, 528 CrossRef CAS PubMed; (b) J. Wilcoxon and B. Abrams, Chem. Soc. Rev., 2006, 35, 1162 RSC; (c) G. V. White, P. Kerscher, R. M. Brown, J. D. Morella, W. McAllister, D. Dean and C. L. Kitchens, J. Nanomater., 2012, 2012, 730746 Search PubMed.
- L. B. D. C. Araujo, S. L. Silva, M. A. M. Galvao, M. R. A. Ferreira, E. L. Araujo, K. P. Randau and L. A. L. Soares, Rev. Bras. Farmacogn., 2013, 23, 736–742 CrossRef CAS PubMed.
- T. S. El-Alfy, S. M. Ezzat and A. A. Sleem, Nat. Prod. Res., 2012, 26, 619–629 CrossRef CAS PubMed.
- H. Kojima, N. Sato, A. Hatanot and H. Ogurat, Phytochemistry, 1990, 29, 2351–2355 CrossRef CAS.
- (a) M. Karlsson, T. Kurz, U. T. Brunk, S. E. Nilsson and C. I. Frennesson, Biochem. J., 2010, 428, 183 CrossRef CAS PubMed; (b) I. L. Jung, PLoS One, 2014, 9, e95492 Search PubMed.
- M. A. Gyamfi, M. Yonamine and Y. Aniya, Gen. Pharmacol., 1999, 32, 661 CrossRef CAS.
- E. A. Elgendy and H. Al-Ghamby, Taiwan Pharm. J., 2007, 59, 113–132 CAS.
- L. M. Schell, K. K. Burnitz and P. W. Lathrop, Ann. Hum. Biol., 2010, 37, 347 CrossRef PubMed.
- O. Lefebvre and R. Moletta, Water Res., 2006, 40, 3671 CrossRef CAS PubMed.
- I. Mohmood, C. B. Lopes, I. Lopes, I. Ahmad, C. Duarte and E. Pereira, Environ. Sci. Pollut. Res. Int., 2013, 20, 1239 CrossRef CAS PubMed.
- C. Tamuly, M. Hazarika, M. Bordoloi and M. R. Das, Mater. Lett., 2013, 4, 102 Search PubMed.
- (a) A. Salem and M. S. El-Maazawi, Chemosphere, 2000, 4, 1173 CrossRef; (b) S. Mowry and P. J. Ogren, J. Chem. Educ., 1999, 76, 970 CrossRef CAS; (c) A. Mills and J. Wang, J. Photochem. Photobiol., A, 1999, 127, 123 CrossRef CAS.
- M. Rafatullah, O. Sulaiman, R. Hashim and A. Ahmad, J. Hazard. Mater., 2010, 177, 70 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Kinetics data, 1H-NMR spectra. See DOI: 10.1039/c4ra10626a |
| ‡ KBAA and SS contributed equally to this work and are listed in alphabetical order. |
| This journal is © The Royal Society of Chemistry 2014 |