 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Smart hydrogels exhibiting UCST-type volume changes under physiologically relevant conditions†
Naohiko
Shimada
a,
Satoru
Kidoaki
b and
Atsushi
Maruyama
*a
aDepartment of Biomolecular Engineering, Tokyo Institute of Technology, 4259 B-57, Nagatsuta, Yokohama 226-8501, Japan. E-mail: amaruyama@bio.titech.ac.jp
bInstitute for Materials Chemistry and Engineering, Kyushu University, Motooka 744-CE11, Nishi-ku, Fukuoka 819-0395, Japan
First published on 9th October 2014
Abstract
In this study, we prepared hydrogels composed of poly(allylurea) copolymers that exhibited UCST-type phase separation behaviour. This is the first example of a hydrogel showing a positive thermo-sensitive volume change under physiologically relevant conditions. Of interest, no hysteresis was observed between cooling and heating swelling changes.
Smart polymers or stimulus-responsive polymers undergo physicochemical changes upon application of an external stimulus. Among them the polymers that exhibit changes under physiologically relevant conditions have been used in biomedical and biological applications. Smart hydrogels based on these polymers are of interest for biomedical technology such as controlled release of drugs1,2 and tissue engineering.3,4 Many smart hydrogels are mainly composed of thermoresponsive polymers that have lower critical solution temperatures (LCST); these include poly(N-isopropylacrylamide) (PNIPAM). Drugs included in this type of hydrogel are released by heating5 because the hydrogels show negative temperature-dependent volume transitions.6
There are few reports describing hydrogels composed of polymers with upper critical solution temperature (UCST) behaviour, because there are few polymers that show UCST-type solution behaviour in aqueous media. The mixture or copolymers of poly(acrylamide) (PAAm) and poly(acrylic acid) (PAAc) exhibit UCST-type phase behaviour in aqueous solution.7–9 Katono et al. successfully prepared interpenetrating polymer network hydrogels composed of PAAm and PAAc.10 The hydrogels showed positive temperature-dependent volume transitions in pure water. Recently, Ning et al. demonstrated that hydrogels composed of poly(N,N-dimethyl(acrylamidopropyl) ammonium propane sulfonate) also show positive thermo-sensitive volume transitions in water.11 These hydrogels do not show such a volume transition under physiological pH and salt conditions, however. The volume transition must occur at physiological pH and in the presence of salt to be useful in biological or biomedical applications. Recently, we reported that ureido-derivatized copolymers, poly(allylurea) (PU) and poly(L-citrulline) copolymers, exhibit UCST-type behaviour under physiological pH and salt conditions.12,13 The phase separation temperature of the polymers could be controlled up to 65 °C by ureido content.
In this study, we prepared and characterized hydrogels consisted of PU copolymers. The poly(allylurea-co-allylamine) (PAU, Fig. 1A) copolymers, PAU83 and PAU86 (ureido contents 83 and 86 mol%, respectively) were prepared by partial ureido-modification of poly(allylamine) hydrochloride (Mw = 15 × 104) as we previously reported.12 First, we prepared hydrogels composed of PAU83 by crosslinking pendent amino groups of the allylamine segments by reductive amination with glutaraldehyde (GA) (see ESI†), because GA crosslinking has been often used for preparing hydrogels14,15 including a poly(allylamine) hydrogel.16 As shown in Fig. 2A, the white gels shrank upon incubation at 6 °C in the buffer. Incubation at 34 °C for 6 h resulted in an increase in transparency and in volume of the gels (Fig. 2B). The rate of the volume change was very slow as shown in Movie S1.† An incubation of 5 to 6 h was necessary to reach constant volume (i.e., equilibrium swelling) of the gels. It was not possible to evaluate precise volume change because gels were too sticky and fragile to handle.
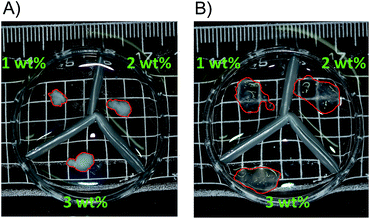 | ||
| Fig. 2 PAU hydrogels at 6 °C (A) and 34 °C (B) cross-linked by glutaraldehyde (1–3 wt%) in 10 mM HEPES (pH 7.5) containing 150 mM NaCl. Red lines trace gel edges. | ||
Hydrogels have been also prepared by photo-irradiation of hydrophilic polymers modified with photo-reactive groups.17–19 The azidophenyl group, for example, releases nitrogen upon UV irradiation forming highly reactive nitrenes which undergo insertion and addition reactions with a wide variety of substrates. Using these reactions for crosslinking purposes, this group has been used to prepare microhydrogels.20–22 Yoshida et al. prepared microhydrogels based on the LCST-type polymer, PNIPAM, modified with azidophenyl groups using a microscope equipped with an objective lens and an Hg lamp.23 They were able to control the shapes of the gels because precise photoirradiation allowed fabrication of gels of particular shapes. Moreover, a rapid LCST-type volume change in response to temperature changes was observed in these microgels. We expected that this UV-crosslinking method allows us to fabricate PAU microgels with well-defined shape and precisely evaluate temperature-dependent behaviour. Also, micro-sized gels could exhibit rapid volume changes in response to temperature alterations because volume change rate of gel is generally proportional to the square of the gel radius.24,25 We synthesized PAU modified with azidophenyl groups (AP-PAU, Fig. 1B) and poly(allylamine) modified with azidophenyl groups (AP-PA) as a control polymer (see ESI†). PAU microgels were obtained by irradiation with UV (330–385 nm) of AP-PAU solution in water. Fig. 3A shows four gels (UV-irradiation for 20 s per gel) in 10 mM HEPES (pH 7.5), 150 mM NaCl at various temperatures. The four microgels examined had round shapes with diameters of 80 μm at 28 °C. Volumes of the gels increased with increasing temperature. The volumes at 60 °C were 9-fold larger than volumes at 28 °C. Transparency also increased with increasing temperature. The control gels prepared by UV-irradiation of AP-PA did not show any morphological changes over the temperature range from 27 to 63 °C (Fig. S1†). As shown in Fig. 3B, the volume of the AP-PAU gels suddenly changed at ∼40 °C. There was no hysteresis in the heating and cooling curves and the volumes did not differ after the heating and cooling cycle (Fig. 3B and Movie S2†). Note that the temperature (∼40 °C) causing the volume change was in good agreement with the phase separation temperature (42 °C, Fig. S2†) of non-irradiated AP-PAU solution. Diameters of gels were increased with increase in UV-irradiation time (Fig. 4). Photo-crosslinking likely occurred in focused area at first. Further irradiation allowed photo-crosslinking at out of focused area, resulting in increase in the diameter. The gels also exhibited temperature positive volume changes (Movie S3†).
 | ||
| Fig. 4 PAU gels at 27 °C (A) and 63 °C (B) prepared by UV irradiation (10–30 s) in 10 mM HEPES (pH 7.5) containing 150 mM NaCl. | ||
To estimate the volume change rate, we evaluated the volume changes in gels when the temperature was rapidly increased or decreased (Fig. 5). During heating, the temperature was increased from 30 to 55 °C within 10 min. Volume changes in the gels were also completed within 10 min. The volume change in gels composed of PNIPAM stops for a certain period of time during the shrinking process because of formation of a hydrophobic skin layer that prevents draining of water from inside gels.26 In contrast, the PAU gels quickly shrank in response to temperature change without this halt in the process. This responsiveness is due to liquid–liquid phase separation mechanism (coacervate formation) of PAU. The gel was highly hydrated even after shrinking, allowing water draining.
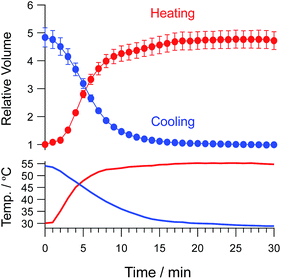 | ||
| Fig. 5 Time course profiles of microgels in response to rapid temperature changes in 10 mM HEPES (pH 7.5), 150 mM NaCl. | ||
In this study, we prepared hydrogels composed of the UCST-type copolymer, PAU, by using two crosslinking methods. These gels exhibited increases in volume and transparency with increasing temperature under physiological pH and salt conditions. In gels prepared by photo-crosslinking, the temperature-induced volume change was rapid. Using the gels fabricated by UV irradiation we were able to quantitatively estimate the volume change as a function of temperature. The thermoresponsive temperature of the gel was almost the same as the phase separation temperature of PAU solution. The phase separation temperature of PAU can be controlled up to 65 °C by changing the ureido content or by modifying the amino groups with other functional groups.12,13 This implies that the thermoresponsive temperature of the gels can also be controlled by use of an appropriate PAU. We believe that gels showing UCST-type volume changes under physiologically relevant conditions will be useful in biomaterials such as temperature-triggered drug release material.
Acknowledgements
Parts of this work were supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Molecular Robotics” (no. 24104003), “Nanomedicine Molecular Science” (no. 2306) and the Cooperative Research Program of “Network Joint Research Center for Materials and Devices” from the Ministry of Education, Culture, Sports, Science and Technology, by Center of Innovation (COI) Program, Japan Science and Technology Agency (JST), and by KAKENHI (no. 23240074, 25350552) from Japan Society for the Promotion of Science.Notes and references
- P. D. Thornton, R. J. Mart and R. V. Ulijn, Adv. Mater., 2007, 19, 1252–1256 CrossRef.
- A. Kikuchi and T. Okano, Adv. Drug Delivery Rev., 2002, 54, 53–77 CrossRef CAS.
- J. D. Kretlow, L. Klouda and A. G. Mikos, Adv. Drug Delivery Rev., 2007, 59, 263–273 CrossRef CAS PubMed.
- R. A. Stile, W. R. Burghardt and K. E. Healy, Macromolecules, 1999, 32, 7370–7379 CrossRef CAS.
- A. S. Hoffman, A. Afrassiabi and L. C. Dong, J. Controlled Release, 1986, 4, 213–222 CrossRef CAS.
- Y. Hirokawa and T. Tanaka, J. Chem. Phys., 1984, 81, 6379–6380 CrossRef PubMed.
- K. Abe, M. Koide and E. Tsuchida, Macromolecules, 1977, 10, 1259–1264 CrossRef CAS.
- E. Tsuchida, K. Abe, E. Tsuchida and K. Abe, Interactions between macromolecules in solution and intermacromolecular complexes, Springer, Berlin, Heidelberg, 1982, pp. 1–130 Search PubMed.
- D. J. Eustace, D. B. Siano and E. N. Drake, J. Appl. Polym. Sci., 1988, 35, 707–716 CrossRef CAS.
- H. Katono, A. Maruyama, K. Sanui, N. Ogata, T. Okano and Y. Sakurai, J. Controlled Release, 1991, 16, 215–227 CrossRef CAS.
- J. Ning, K. Kubota, G. Li and K. Haraguchi, React. Funct. Polym., 2013, 73, 969–978 CrossRef CAS PubMed.
- N. Shimada, H. Ino, K. Maie, M. Nakayama, A. Kano and A. Maruyama, Biomacromolecules, 2011, 12, 3418–3422 CrossRef CAS PubMed.
- N. Shimada, M. Nakayama, A. Kano and A. Maruyama, Biomacromolecules, 2013, 14, 1452–1457 CrossRef CAS PubMed.
- G. A. F. Roberts and K. E. Taylor, Die Makromolekulare Chemie, 1989, 190, 951–960 CrossRef CAS.
- M. George and T. E. Abraham, Int. J. Pharm., 2007, 335, 123–129 CrossRef CAS PubMed.
- G. V. R. Rao, T. Konishi and N. Ise, Macromolecules, 1999, 32, 7582–7586 CrossRef CAS.
- H. Okino, Y. Nakayama, M. Tanaka and T. Matsuda, J. Biomed. Mater. Res., 2002, 59, 233–245 CrossRef CAS PubMed.
- T. Kawano and S. Kidoaki, Biomaterials, 2011, 32, 2725–2733 CrossRef CAS PubMed.
- S.-H. Kim and C.-C. Chu, J. Biomed. Mater. Res., Part B, 2009, 91, 390–400 CrossRef PubMed.
- S.-H. Park, S.-Y. Seo, J.-H. Kang, Y. Ito and T.-I. Son, J. Appl. Polym. Sci., 2013, 127, 154–160 CrossRef CAS.
- T. Sugawara and T. Matsuda, Macromolecules, 1994, 27, 7809–7814 CrossRef CAS.
- W. Lequieu, N. I. Shtanko and F. E. Du Prez, J. Membr. Sci., 2005, 256, 64–71 CAS.
- R. Yoshida, K. Omata, K. Yamaura, M. Ebata, M. Tanaka and M. Takai, Lab Chip, 2006, 6, 1384–1386 RSC.
- T. Tanaka and D. J. Fillmore, J. Chem. Phys., 1979, 70, 1214–1218 CrossRef CAS PubMed.
- T. Tanaka, E. Sato, Y. Hirokawa, S. Hirotsu and J. Peetermans, Phys. Rev. Lett., 1985, 55, 2455–2458 CrossRef CAS.
- E. Sato Matsuo and T. Tanaka, J. Chem. Phys., 1988, 89, 1695–1703 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, movies of PAU and PAA hydrogels, transmittance curve of AP-PAU. See DOI: 10.1039/c4ra10612a |
| This journal is © The Royal Society of Chemistry 2014 |


