A versatile ambient pressure drying approach to synthesize silica-based composite aerogels
Jin Wanga,
Yong Weia,
Weina Hea and
Xuetong Zhang*ab
aSuzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou, 215123, P. R. China
bSchool of Materials Science and Engineering, Beijing Institute of Technology, Beijing, 100081, P. R. China. E-mail: zhangxtchina@yahoo.com; xtzhang2013@sinano.ac.cn
First published on 26th September 2014
Abstract
A versatile ambient pressure drying (APD) method for synthesizing silica aerogels, as well as silica-based composite aerogels with different colors or functions, has been developed in this work. The presented polyethoxydisiloxane (PEDS) was held under basic conditions in ethanol and alcogels were obtained, which was followed by hexane solvent-exchange and pore surface modification, and then the wet gels were dried under ambient pressure at temperatures ranging from 25 to 150 °C to produce silica aerogels with high BET surface areas and low thermal conductivities. Various guests were introduced, e.g., dyes, Fe3O4 nanoparticles and Ag nano-wires in the sol–gel process, and colored, magnetic or bio-functional silica aerogels were synthesized by the APD method. All the products were of high BET surface areas varying from 500 to 1000 m2 g−1 and of average pore sizes in the range of 8–20 nm, regardless of the drying temperature and the presence of dye molecules or embedded nano-particles. The tapping densities of the aerogels were in the range of 0.03 to 0.2 g cm−3, and the thermal conductivities were found varying from 0.022 to 0.040 W m−1 K−1. Moreover, the structures and morphologies of the aerogels were investigated by FTIR and SEM, respectively. The stability of the colored aerogels was studied by UV-vis spectrometry, and the results indicate that the dyes were embedded in the aerogels and may be gradually released. Furthermore, the capacity of the magnetic aerogels for wastewater treatment was investigated. In general, an APD method, involving a single hexane-exchange step, has been developed, and the preparation of various silica-based composite aerogels prepared by APD is achieved. This APD method shows a great potential to synthesize silica aerogels and its hybrid aerogels on an industrial scale.
1. Introduction
Microporous and mesoporous materials have attracted extensive interest from both academia and industry due to their structural properties such as high porosity and high specific surface areas. They are widely used as drug delivery systems,1 tissue scaffolds,2 and for filtration,3 catalyst loading,4 energy storage,5 and thermal insulation.6 Aerogels are the most highly porous materials that are currently available. In addition to the high porosity (∼98%), aerogels also have a large surface area (up to 1200 m2 g−1), low density (down to 0.003 g cm−3), and extremely low thermal conductivity (∼0.012 W m−1 K−1).7,8 Though silica aerogels were first discovered by Kistler9,10 in the early 1930s, it is only recently that silica aerogels have been attracting growing interest, especially in the area of large scale manufacture of silica aerogels due to the advantages mentioned above and the wide applicability in thermal insulation.11–17Aerogels are normally synthesized by the supercritical extraction of the pore liquid from wet gels, which is expensive, and it is hard to fabricate large dimensional aerogels in this way; therefore, industrial-scale production of aerogels is restricted. In order to solve these problems, the ambient pressure drying (APD) method has been proposed. The APD method occurs via solvent-exchange with low surface tension solvents and surface modification of the wet gels,18 in which the silylated surfaces do not participate in condensation reactions or hydrogen bonding as the gel is collapsed by the capillary tension developed during drying. Therefore, as the liquid–vapour recedes into the gel interior, the shrunken elastic network progressively springs back toward its original porous state. However, solvent-exchange is a lengthy and tedious process,19,20 which usually takes several days or weeks. Recently, economic and large-scale industrial production of silica aerogels by APD has been developed and the time of production has been remarkably reduced to one day.21,22 Nevertheless, to our best knowledge, all the aerogels prepared by APD involve a large amount of water washing or proton exchange, ethanol wash or exchange, and finally hexane exchange and modification, all of which consumes considerable amount of solvent and more solvent-exchange steps are involved, which increases the possibility of environmental pollution. Therefore, methods that avoid water-washing and multiple steps of solvent-exchange in synthesis of aerogels, is an attractive topic. For instance, there is interest in developing a method that only involves hexane exchange and modification (no water wash and ethanol exchange), which may not only considerably reduce the fabrication time and impact on environment, but also reduce the fabrication cost.
Moreover, composite and functional aerogels are another interesting topic. For example, aerogels that are a composition of silica and polymers can have significantly improved mechanical properties as compared to pure silica aerogels,23,24 and the composition of silica with TiO2 and B4C can reduce the thermal conductivity of silica aerogels by the prevention of thermal radiation.25 The composite aerogels, however, were normally prepared by a supercritical liquid drying (SCD) method. The synthesis of silica-based composite aerogels and functional aerogels via APD method is rare; one example is the synthesis of titania-silica aerogel-like microspheres by a water-in-oil emulsion method reported by Gan et al.26 In order to fulfill the industrial scale production of composite aerogels, neither the SCD nor the present APD technology is desirable because the SCD limits the large scale fabrication, whereas the present APD involves washing with water and ethanol exchange, which may wash out the composite components especially when the hybrids are dyes.
In order to solve the abovementioned problems, in the present work, we developed a simple APD method to synthesize silica aerogels and its composite aerogels without any water-wash and ethanol solvent-exchange. As illustrated in Scheme 1, polyethoxydisiloxane (PEDS), which can be easily prepared from tetraethoxysilane (TEOS),27–31 was used as the precursor for three reasons: (1) PEDS can undergo direct gelation in alcohol under basic conditions; therefore, the alcogels can directly solvent-exchange with hexane; (2) there are many ethoxy groups on the backbone, which can significantly reduce the amount of modification reagents (such as trimethylchlorosilane (TMCS) and hexamethyldisilazane (HMDS)); and (3) by introducing dyes and other components in the sol–gel process, composite silica aerogels can be produced by the APD method on a large scale.
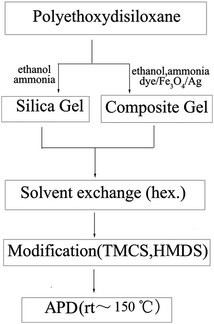 | ||
| Scheme 1 A flowchart showing experimental procedures for the synthesis of silica aerogels and silica-based composite aerogels. | ||
Thus, silica aerogels were synthesized by using TMCS and HMDS as the modification reagents via the APD method proposed in Scheme 1. Moreover, the impacts of drying temperatures on the structure and physical properties of the aerogels were also investigated. Applying the same APD method, various silica-based organic and inorganic composite aerogels showing different colors or functions were synthesized. For example, methyl orange, rhodamine B, victoria blue B, CuCl2, Fe3O4, and Ag nano-wires were successfully embedded in the silica aerogels. FTIR, thermal conductivity, and BET measurements were conducted, and the results indicate that the BET surface areas and thermal conductivity of the composite aerogels were not significantly affected, but even improved.
2. Experimental
2.1. Materials
TEOS, methyl orange, rhodamine B, victoria blue B (Aladdin Industrial Corporation), and TMCS, HMDS (TCI) are used as received. Nano Fe3O4 particles with average diameter of 20 nm were used (Aladdin Industrial Corporation). Ag nano-wires were prepared according to the literature32 and used in an ethylene glycol solution, which was stabilized by polyvinylpyrrolidone (PVP). PEDS was prepared according to the literature,28 and the molar ratio of TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol is 1
ethanol is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3
1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5. Other reagents are of analytical purity and used as received.
2.5. Other reagents are of analytical purity and used as received.
2.2. Synthesis of silica aerogels by APD
Silica aerogels were prepared according to Scheme 1. By using the sample shown in Table 1 (entry 3) as an example: PEDS (30 g) was dissolved in 200 ml ethanol and stirred in a 500 ml flask for 10 min, and then 500 μl of ammonium hydroxide was added drop-by-drop into the PEDS solution and gelation was obtained in 30 min. After aging at 40 °C for 2 h, the alcogel was crushed and vigorously stirred in 200 ml hexane for 5 h. The gels were collected by filtration or centrifugation and again vigorously stirred in 200 ml hexane for another 5 h. Finally, the gels were modified by TMCS (5 ml) in 100 ml hexane for 2 h and dried after filtration at 150 °C for 30 min.| Entrya | Modification reagentb | Drying temperature (°C) | Density (mg cm−3) | Surface areas (m2 g−1) | Average pore diameter (nm) | Pore volume (cm3 g−1) | Thermal conductivity (W m−1 K−1) |
|---|---|---|---|---|---|---|---|
| a 30 g PEDS dissolved in 200 ml ethanol as determined from the feed molar ratio of TEOS.b 5% (in volume) of TMCS or HMDS in hexane. | |||||||
| 1 | TMCS | 25 | 69.0 | 880.23 | 9.4 | 2.18 | 0.0240 |
| 2 | TMCS | 80 | 36.7 | 905.81 | 11.6 | 2.50 | 0.0241 |
| 3 | TMCS | 150 | 58.5 | 925.01 | 13.5 | 2.73 | 0.0237 |
| 4 | HMDS | 150 | 34.1 | 835.52 | 10.1 | 2.45 | 0.0241 |
2.3. Synthesis of colored silica aerogels by APD
The colored composite silica aerogels were synthesized via the APD method, in the same way to that described above for the pure silica aerogels. The only difference is the addition of a small amount of dye or metal salt into the PEDS solution before gelation.2.4. Synthesis of magnetic silica aerogels and Ag nano-wire–silica composite aerogels via APD
Functional silica aerogels were also synthesized via the APD method as described in Section 2.2. Similar to the method of synthesis of colorful aerogels, Fe3O4 nano-particles and Ag nano-wires were added during the sol–gel process. It should be noted that, for synthesizing Fe3O4–silica composite gels, more than 2 ml ammonium hydroxide must be added. The gelation occurred under stirring such that the Fe3O4 nano-particles can be uniformly dispersed in the silica networks; moreover, HMDS should be used as the modification reagent.2.5. Characterization
The pore size distributions and average pore diameters of the aerogels were analyzed by the BJH nitrogen adsorption and desorption method (ASAP 2020, Micromeritics, USA). The surface areas of the aerogels were determined by the Brunauer–Emmett–Teller (BET) method, based on the amount of N2 adsorbed at pressures 0.05 < P/P0 < 0.3. The cumulative pore volume was measured at the point P/P0 = 0.99. The FTIR spectra were measured on a Thermo Scientific Nicolet iN10 spectrometer using the transmission mode. The UV-vis spectra were determined on a UV-1800 instrument, and the scanning range was from 300 to 800 nm. The thermal conductivity of the aerogel powders under room temperature was measured by using the transient hot wire method (XIATECH C2000, China), the data was collected three times with 5 min intervals between each measurement. The micro-structural studies of some selected aerogels were performed using field-emission scanning electron microscopy (Quanta 400 FEG). The samples were coated with Au nano-powder under a current of 20 mA for 1 min. TEM measurement was carried out on a Tecnai G2 F20 S-TWIN instrument. Samples were prepared by dispersing aerogel powders in ethanol and dropping onto a copper grid, and finally it was dried under air for one week. The aerogels densities reported are the tapping densities in this work.3. Results and discussion
3.1. The synthesis of silica aerogels and their physical properties
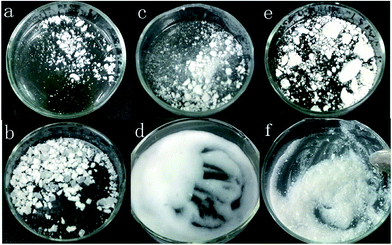
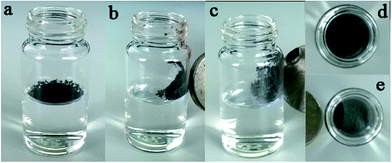
The APD method to synthesize silica aerogels has been developed contrary to the supercritical liquid drying method, in which the supercritical conditions are not needed and the ambient pressure is enough due to the spring-back effects.18 In order to scale-up the synthesis of aerogels for industrial production, sodium silicate has been widely used as a precursor due to its lower cost as compared to TEOS and trimethoxysilane (TMOS).21,22 However, tedious water-wash steps need be carried out when sodium silicate is used, and before solvent exchange with hexane, alcohol exchange for the water is required. Moreover, a large amount of modification reagents such as TMCS is essential (side reactions between TMCS and alcohol and water are significant). These processes increase the fabrication costs and time, in addition to the risk for pollution. In order to simplify the approach for large scale production of silica aerogels, PEDS was used as a precursor because it is commercially available and can be easily prepared from TEOS. More importantly, as illustrated in Scheme 1, no water-wash and alcohol exchange was needed. The wet-gels can be directly exchanged with hexane and modified with a very small amount of TMCS or HMDS because there are plenty of hydrophobic –OCH2CH3 groups on the Si backbone, rather than the –OH groups (This can be confirmed by FTIR spectrometry and will be discussed in the following Section). By introducing dyes or other functional components in the sol–gel process, composite silica aerogels would be synthesized by this APD method.To confirm this proposal, silica aerogels were prepared by a sol–gel process from PEDS, and after aging for ca. 2 h, the gel was solvent-exchanged with hexane and modified with TMCS or HMDS. Finally, the modified gels were dried at room temperature or 150 °C under ambient pressure. As a control, xerogel was prepared via the same method without modification, and the products are shown in Fig. 1. Silica aerogels were successfully synthesized for the samples modified with a small amount of TMCS or HMDS (Fig. 1c–f), whereas stiff, glass-like xerogels were formed for the samples without modification (Fig. 1a and b). Moreover, as can be seen in Fig. 1, the aerogels dried at 150 °C formed liquid-like powders, while those dried at room temperatures are small bricks but can be easily smashed into liquid-like powders. Thus, silica aerogels are prepared by the simplified APD, even under ambient pressure and ambient temperatures.
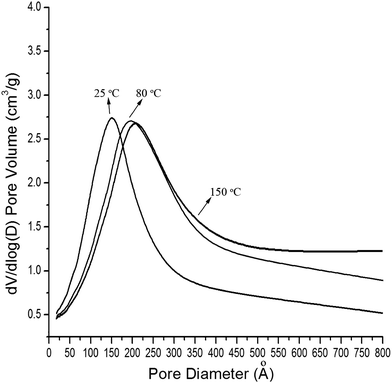
In order to investigate the effects of the drying temperature on the structure and physical properties of aerogels, three batches of aerogels were prepared from the same modified wet gels by drying at 25, 80 and 150 °C, respectively. Their tapping densities, BET surface areas, average pore diameters, pore volume and thermal conductivities were characterized and the results are presented in Table 1 (entry 1–3). The N2 adsorption–desorption isotherms of the aerogels are shown in Fig. 2, and the pore size distribution curves are given in Fig. 3. As can been seen, the physisorption isotherms obtained for all the silica aerogels exhibit hysteresis loops, which are due to the characteristic features of mesoporous materials (type IV isotherms). From Table 1, we can conclude that the BET surface areas, average pore diameters and pore volumes are all increased with increase in drying temperature, which may be possibly due to a better spring-back effect under higher temperature.18 High BET surface areas of 925.01 m2 g−1 and very low thermal conductivity of 0.0237 W m−1 K−1 was achieved when the silica aerogels were dried at 150 °C. Interestingly, high BET surface areas of 880.23 m2 g−1 and very low thermal conductivity of 0.0240 W m−1 K−1 was obtained even when the silica aerogel was dried at room temperature. The properties of silica aerogel modified by HMDS and dried at 150 °C are shown in Table 1. The low tapping density (34.1 mg cm−3), high surface areas (835.52 m2 g−1) and high pore volume (2.45 cm3 g−1) indicated that the silica aerogels can also be prepared by using HMDS as the modification reagent.
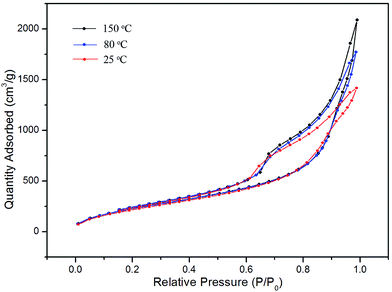 | ||
| Fig. 2 N2 adsorption–desorption isotherms of silica aerogels via APD at indicated temperatures (entry 1–3 presented in Table 1). | ||
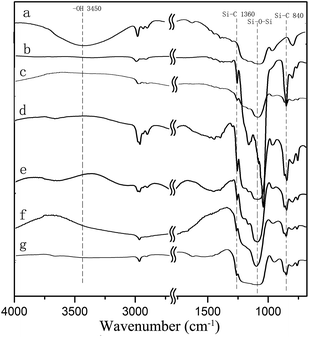
The chemical structures of the aerogels were confirmed by FTIR spectra as shown in Fig. 4. Xerogel (Fig. 1b) synthesized without modification (Fig. 4a) exhibits a broad absorption peak at 3450 cm−1, corresponding to the vibrations of –OH. Moreover, the strong peaks located at 2790 to 2980 cm−1 demonstrated that many –OCH2CH3 groups exist in the system. As a result, a relatively small amount of modification reagents can work well (5% in volume). Fig. 4b and c show the FTIR spectra of silica aerogels modified with TMCS (entry 3) and HMDS (entry 4), respectively. There are two peaks appearing at 840 and 1360 cm−1 as compared to Fig. 4a, which correspond to the vibrations of Si–C. Moreover, the –OH peak at 3450 cm−1 is almost unobservable in the silica aerogels. These results indicate that the –OH groups have been successfully replaced by –Si(CH3)3.
3.2. Colored silica aerogels and dye releasing behaviors
Because there is no water-wash and alcohol exchange in the simplified APD method, colorful silica aerogels may be produced by the APD method based on the fact that dyes or metal ions can be dissolved in ethanol but may insoluble in hexane or cannot be completely washed out by hexane-exchange after embedding in the silica networks. Therefore, CuCl2, methyl orange, rhodamine B, and victoria blue B were added in the sol–gel process to synthesize colored aerogels as shown in Scheme 1. The images of the colored silica aerogels are shown in Fig. 5, and their structure and physical properties are summarized in Table 2. CuCl2, methyl orange, rhodamine B, and victoria blue B–silica composite aerogels are shown in yellow, light pink, bright pink, and blue, respectively. Though 5 w/w% of the dyes are embedded in the aerogels, the tapping density, BET surface areas, and thermal conductivities are not apparently affected. For instance, the thermal conductivities of colored aerogels are lower than 0.030 W m−1 K−1, except for CuCl2 composite aerogels, while the BET surface area of the methyl orange–silica composite aerogels is 931.62 m2 g−1. The pore diameters of the aerogels are smaller than 20 nm. When compared to the pore volumes of pure silica aerogels presented in Table 1, extremely high pore volumes are obtained for the organic dye composite silica aerogels, from 3.05 to 3.53 cm3 g−1 of the victoria blue B-composite aerogels and the methyl orange-composite aerogels, respectively. The reason is not very clear at present, but from the pore distribution curves shown in Fig. 3 and 6, broad pore distributions and large pore diameters were observed in the dye-composite aerogels, which may contribute to a relatively larger pore volume.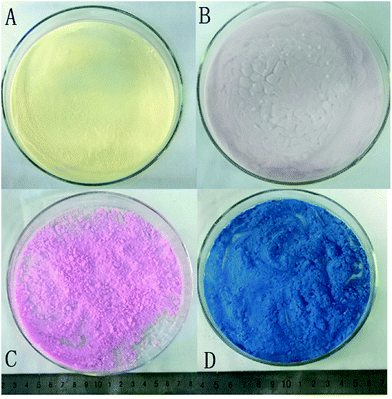 | ||
| Fig. 5 Images of colored aerogel powers: (A) CuCl2–silica, (B) methyl orange–silica, (C) rhodamine B–silica, and (D) victoria blue B–silica composite aerogels. | ||
| Entrya | Dye/metallic oxide | Density (mg cm−3) | Surface areas (m2 g−1) | Average pore diameter (nm) | Pore volume (cm3 g−1) | Thermal conductivity (W m−1 K−1) |
|---|---|---|---|---|---|---|
| a The drying temperature is 150 °C, the modification reagent is TMCS, and the amount of dye to silica is 5% by weight. | ||||||
| 5 | CuCl2 | 105.0 | 736.76 | 8.7 | 1.70 | 0.0356 |
| 6 | Methyl orange | 58.6 | 931.62 | 17.8 | 3.54 | 0.0221 |
| 7 | Rhodamine B | 58.1 | 850.35 | 11.2 | 3.05 | 0.0248 |
| 8 | Victoria blue B | 49.5 | 910.01 | 9.2 | 3.23 | 0.0298 |
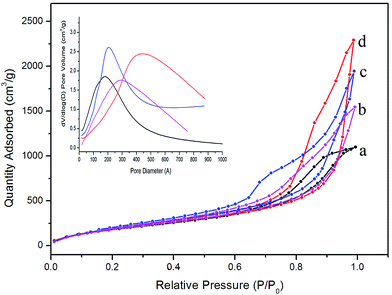 | ||
| Fig. 6 N2 adsorption–desorption isotherms of colored silica aerogels: (a) entry 5, (b) entry 7, (c) entry 8, and (d) entry 6. The inset is the pore size distribution of the corresponding aerogels. | ||
Fig. 4d and e show the FTIR spectra of the pink (entry 7) and blue (entry 8) aerogels, respectively. Similar to the peaks of pure silica aerogels in entry 3 and 4, two peaks appearing at 840 and 1360 cm−1, which can be attributed to the fact that the vibrations of Si–C have been observed. Moreover, the –OH peak at 3450 cm−1 is almost unobservable in the colored silica aerogels. These results indicate that the existence of dyes does not prohibit the modification process. The N2 adsorption–desorption isotherms of the colored aerogels shown in Fig. 6 exhibit similar type IV isotherms with hysteresis loops similar to that of pure silica aerogels (entry 1–3), which indicates that the dye molecules may not form large particles that block the mesoporous holes. The SEM image (Fig. 7) shows the micro-morphology of the colored aerogels. Uniform nano-particle networks can be clearly seen from Fig. 7A (blue aerogel), which is coincident with the narrow pore size distribution as shown in Fig. 6 (inset figure, blue line). In the SEM images of the rhodamine B–silica composite aerogels (Fig. 7B) and methyl orange–silica composite aerogels (Fig. 7C), nano-particles can also be observed. However, the pores are not as uniform as that victoria blue B–silica aerogels. The wider pore size distributions of two aerogels are obvious as shown in Fig. 6 (inset figure, red and pink line).
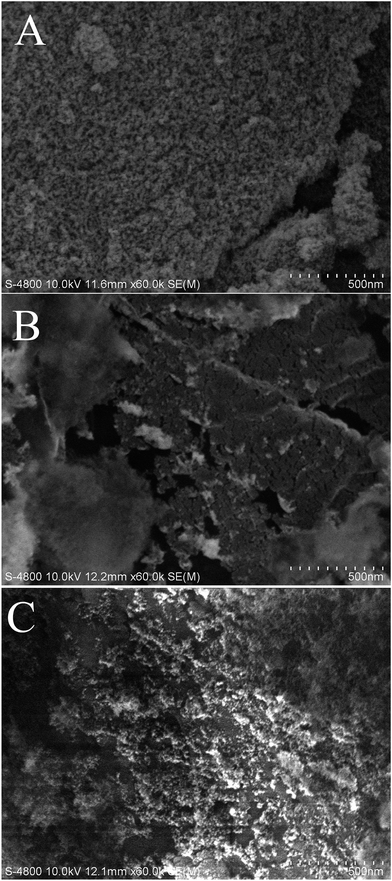 | ||
| Fig. 7 SEM images of (A) victoria blue B–silica composite aerogel, (B) rhodamine B–silica composite aerogel, and (C) methyl orange–silica composite aerogel. | ||
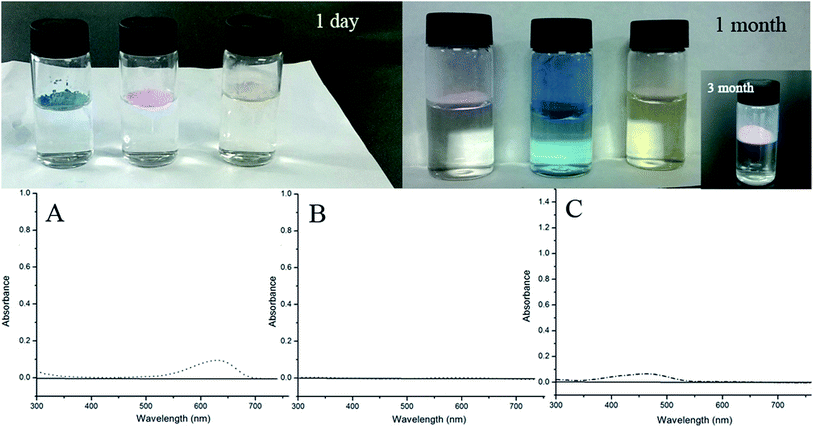
The stability of the colored aerogels was studied by UV-vis spectrometry by suspending the colored aerogels in distilled water. As shown in the images in Fig. 8, the aerogels are hydrophobic and the dyes cannot be washed out even under vigorous stirring in the first few days. However, after continuous immersing in water for one month, victoria blue B and methyl orange were slightly released into the water, as can be seen in the image on the top-right site of Fig. 8. The UV-vis spectra shown in Fig. 8A and C further confirm that a small amount of victoria blue B and methyl orange has been released into the water where aerogels were suspended. Interestingly, the rhodamine B composite aerogels are much more stable than the other ones as indicated by both the images and UV-vis spectrum (Fig. 8B); furthermore, no trace of the release of rhodamine B was observed. Moreover, after three months of exposure to light and air, there was still no trace of the release of rhodamine B (inset figure in Fig. 8).
3.3. Magnetic silica aerogels and their potential application for oil purification
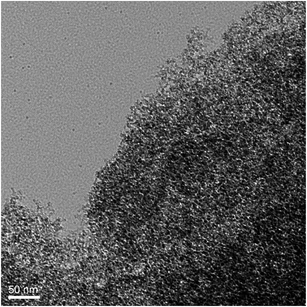
Based on the successful synthesis of colored silica aerogels, we wondered if other functional silica aerogels would also be synthesized by this APD method. Therefore, the synthesis of magnetic silica aerogels, Fe3O4 nano-particles–silica composite aerogels, was investigated. However, two problems were soon recognized: (1) Fe3O4 is insoluble in ethanol and it is hard to be homogeneously disperse into the wet-gel because the gels are usually formed by standing; and (2) when modified with TMCS, Fe3O4 particles were etched and Fe3O4–silica composite aerogels cannot be produced. In order to solve these problems, a relatively larger amount of ammonium hydroxide was added to the sol–gel process to let the gelation occur under vigorous stirring. After solvent-exchange with hexane, HMDS was used as the modification reagent. In these conditions, magnetic silica aerogels were successfully prepared by the APD method. These aerogels can be seen in the image shown in Fig. 9. The structure of the aerogels is confirmed by FTIR spectra shown in Fig. 4g. Similar to the FTIR spectra of the silica aerogels and dye–silica composite aerogels, the Fe3O4–silica composite aerogels also exhibit two peaks at 840 and 1360 cm−1, which correspond to the vibrations of Si–C. Moreover, the –OH peak at 3450 cm−1 is almost unobservable in the magnetic silica aerogels. These results clearly indicate that HMDS works efficiently for the APD of functional silica aerogels.A detailed investigation of the impacts of the contents of Fe3O4 and drying temperature on the structure and physical properties of the composite aerogels was undertaken. As presented in Table 3, two samples containing 2 wt% of Fe3O4 were dried at 80 and 150 °C, respectively, whereas another two samples containing 5 wt% of Fe3O4 were also dried at 80 and 150 °C, respectively. As can be seen in Table 3, the average pore diameter and pore volume increased with higher drying temperature, whereas the density and thermal conductivity decreased, similar to that of pure silica aerogels; however, the BET surface areas are slightly decreased. Nevertheless, the values are comparable to that of silica aerogels and dye-composite aerogels, which indicates that the Fe3O4 nano-particles are successfully embedded in the silica networks. Moreover, the TEM image of composite aerogels (entry 10) shown in Fig. 10 suggests that the Fe3O4 nano-particles are uniformly dispersed in the silica framework. As can be seen in Fig. 10, the framework of the composite aerogels is made up nano particle networks, and no obvious large aggregation domains have been observed.
| Entrya | Drying temperature (°C) | Density (mg cm−3) | Surface areas (m2 g−1) | Average pore diameter (nm) | Pore volume (cm3 g−1) | Thermal conductivity (W m−1 K−1) |
|---|---|---|---|---|---|---|
| a The modification reagent is HMDS.b The feed weight ratio of Fe3O4 to silica is 2%.c The feed weight ratio of Fe3O4 to silica is 5%. | ||||||
| 9b | 80 | 95.2 | 695.27 | 10.3 | 2.02 | 0.0257 |
| 10b | 150 | 78.1 | 679.76 | 14.6 | 2.72 | 0.0250 |
| 11c | 80 | 106.0 | 563.40 | 13.7 | 2.22 | 0.0294 |
| 12c | 150 | 83.0 | 542.19 | 18.27 | 2.79 | 0.0260 |
The N2 adsorption–desorption isotherms and pore size distribution of Fe3O4–silica composite aerogels are given in Fig. 11. All the samples exhibit type IV isotherms with hysteresis loops similar to that of pure silica aerogels, and the results indicate that the shape of the pores of these composite aerogels depend on the PEDS precursors while the embedded components are not critical for the pore shapes. This conclusion can be further confirmed by the isotherm traces of the dye-composite aerogels, as well as that of the Ag-composite aerogels, which will be discussed in the next section.
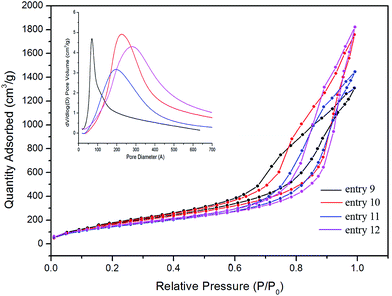 | ||
| Fig. 11 N2 adsorption–desorption isotherms of Fe3O4–silica composite aerogels. The inset is the pore size distribution of the corresponding aerogels. | ||
Due to the interesting magnetic properties of the Fe3O4–silica composite aerogels, as well as their hydrophobicity and high pore volume, the wastewater treatment capacity of the aerogels was briefly investigated in this work. As a preliminary study, the adsorption of hexane by the composite aerogels was carried out. When 18.4 mg of aerogels was used, up to 191.8 mg hexane can be adsorbed, which indicated that the adsorption capacity of the aerogels to hexane was more than 10 times by weight. Interestingly, as the aerogels are magnetic, they can be easily collected under a magnetic field after adsorption of hexane (Fig. 12). Furthermore, while the aerogels were dispersed in a large amount of hexane, it could be simply aggregated and collected by using a magnetic field.
3.4. Ag nano-wire–silica composite aerogels and their physical properties
The versatility of the APD method has been proven by synthesizing various organic and inorganic composite silica aerogels, such as organic small molecules, metal ions, and nano-particles, which were all embedded in the silica networks by the APD method (only hexane-exchange and surface modification). In this section, we further demonstrate that nano-wires can also be embedded in the silica networks. Ag nano-wires were used because we expected that functional composite silica aerogels, such as electron conductivity and sterilization, could be achieved. It should be noted that the relatively mild HMDS should be used as the modification reagent, as TMCS can etch the Ag nano-wires due to the formation of HCl.The impact of the contents of Ag nano-wire and drying temperature on the structure and physical properties of the composite aerogels was investigated. As shown in Table 4, all the samples show large BET surface areas higher than 640 m2 g−1 and pore volumes larger than 1.2 cm3 g−1, despite the introduction of Ag nano-wires. However, the tapping densities (>100 g cm−3) and thermal conductivities (mostly >0.03 W m−1 K−1) of these aerogels are higher than those of silica aerogels and dye, as well as Fe3O4 composite silica aerogels. This may be due to that the fact that a higher content of Ag was used (8%), and the PVP surfactants for the uniform distribution of Ag nano-wires were not washed out by hexane exchange. Nevertheless, the structure of the Ag–silica composite aerogels is not much affected.
| Entrya | Drying Temperature (°C) | Density (mg cm−3) | Surface areas (m2 g−1) | Average pore diameter (nm) | Pore volume (cm3 g−1) | Thermal conductivity (W m−1 K−1) |
|---|---|---|---|---|---|---|
| a The modification reagent is HMDS.b The feed weight ratio of Ag to silica is 5%.c The feed weight ratio of Ag to silica is 8%. | ||||||
| 13b | 80 | 148.2 | 722.49 | 8.76 | 1.83 | 0.0347 |
| 14b | 150 | 105.3 | 734.38 | 12.89 | 2.67 | 0.0270 |
| 15c | 80 | 194.0 | 667.0 | 6.64 | 1.23 | 0.0383 |
| 16c | 150 | 162.7 | 642.62 | 9.16 | 1.69 | 0.0321 |
The FTIR spectrum of Ag–silica aerogels (entry 14) shown in Fig. 4f exhibits two peaks at 840 and 1360 cm−1, which correspond to the vibrations of Si–C, while the –OH peak at 3450 cm−1 is almost unobservable. This indicated that the –OH has been replaced by –Si(CH3)3 groups. The N2 adsorption–desorption isotherms and pore size distribution of Ag–silica composite aerogels are presented in Fig. 13. All the samples exhibit type IV isotherms with hysteresis loops similar to that of the silica-based composite aerogels.
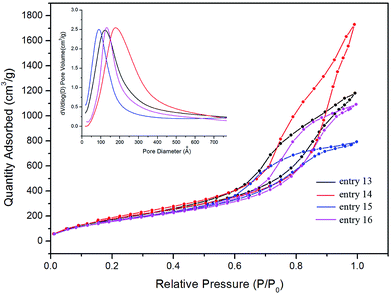 | ||
| Fig. 13 N2 adsorption–desorption isotherms of Ag–silica composite aerogels. The inset is the pore size distributions of the corresponding aerogels. | ||
Though the Ag–silica composite aerogels are successfully synthesized, the electron-conducting measurement (not shown) indicates that the aerogels are an electron insulator. We considered that the Ag content is low in the composite aerogels and no connecting networks of Ag nano-wires exist in the aerogels; therefore, the composite aerogels is electron insulating. To confirm this speculation, SEM measurement was performed. Fig. 14 shows the images of the Ag composite aerogels (entry 14), in which the Ag nano-wires can be clearly identified (red circle) and are dispersed and embedded in the silica matrix. Moreover, no Ag network is found and the composite aerogels is not electron-conducting.
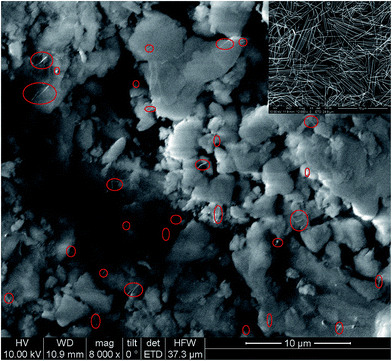 | ||
| Fig. 14 SEM image of Ag nano-wire–silica composite aerogel (inset is the SEM image of Ag nano-wire, Ag networks are formed by contacting and twining). | ||
4. Conclusions
A versatile APD method of synthesizing silica aerogels was developed in this work, in which only hexane solvent-exchange was performed and a small amount of modification reagents were used. Moreover, drying temperature ranged from room temperature to 150 °C; thus, the ambient pressure and ambient temperature drying method is also reported. This APD method is capable of synthesizing various composite silica aerogels, showing different colors and functions. All the silica and silica-based composite aerogels show high BET surface areas ranging from 500 to 1000 m2 g−1 and average pore sizes in the range of 8–20 nm, regardless of the drying temperature, the dye or embedded nano-particles. The tapping densities of the aerogels were in the range of 0.03 g cm−3 to 0.2 g cm−3, and the thermal conductivities were found to vary from 0.0221 to 0.04 W m−1 K−1. In addition, larger pore diameters can be achieved by increasing the drying temperature, while the pore shape of the composite aerogels was found to depend on the precursors used rather than the embedded components (such as organic dyes and metal nano-particles). The UV-vis measurement of colored silica aerogels indicates that the dyes are stable, and may be released rather slowly when continuously immersed in water for one month. The capacity of the magnetic aerogels for hexane adsorption is higher than 10 times by weight, and the aerogels can be easily collected under a magnetic field. In general, a versatile APD method was developed, and due to the simplified process with only hexane-exchange and the ability to synthesize various composite aerogels, this APD method is especially useful for the industrial large-scale production of silica aerogels.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21373024), the Innovation Program of the Beijing Institute of Technology and the 100 Talents Program of the Chinese Academy of Sciences.References
- J. Fu, Y. Zhu and Y. Zhao, J. Mater. Chem. B, 2014, 2, 3538–3548 RSC.
- R. Ravichandran, D. Sundaramurthi, S. Gandhi, S. Sethuraman and U. M. Krishnan, Microporous Mesoporous Mater., 2014, 187, 53–62 CrossRef CAS PubMed.
- Y. Yamauchi and T. Kimura, Chem. Commun., 2013, 49, 11424–11426 RSC.
- M. Wang, X. Wang, Q. Yue, Y. Zhang, C. Wang, J. Chen, H. Cai, H. Lu, A. A. Elzatahry, D. Zhao and Y. Deng, Chem. Mater., 2014, 26, 3316–3321 CrossRef CAS.
- N. Linares, A. M. Silvestre-Albero, E. Serrano, J. Silvestre-Albero and J. Garcia-Martinez, Chem. Soc. Rev., 2014 10.1039/c3cs60435g.
- T. J. Ha, H. H. Park, H. W. Jang, S. J. Yoon, S. Shin and H. H. Cho, Microporous Mesoporous Mater., 2012, 158, 123–128 CrossRef CAS PubMed.
- P. B. Sarawade, J. K. Kim, A. Hilonga and H. T. Kim, Solid State Sci., 2010, 12, 911–918 CrossRef CAS PubMed.
- A. V. Rao, N. D. Hegde and H. Hirashima, J. Colloid Interface Sci., 2007, 305, 124–132 CrossRef PubMed.
- S. S. Kistler, Nature, 1931, 127, 741 CrossRef CAS.
- S. S. Kistler, J. Phys. Chem., 1932, 36, 52–64 CrossRef CAS.
- D. M. Smith, A. Maskara and U. Boes, J. Non-Cryst. Solids, 1998, 225, 254–259 CrossRef CAS.
- L. Kocon, F. Despetis and J. Phalippou, J. Non-Cryst. Solids, 1998, 225, 96–100 CrossRef CAS.
- A. Soleimani Dorcheh and M. H. Abbasi, J. Mater. Process. Technol., 2008, 199, 10–26 CrossRef CAS PubMed.
- Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer, 2011 Search PubMed.
- R. Baetens, B. P. Jelle and A. Gustavsen, Energ. Build., 2011, 43, 761–769 CrossRef PubMed.
- D. Sanli and C. Erkey, ACS Appl. Mater. Interfaces, 2013, 5, 11708–11717 CAS.
- J. C. H. Wong, H. Kaymak, S. Brunner and M. M. Koebel, Microporous Mesoporous Mater., 2014, 183, 23–29 CrossRef CAS PubMed.
- S. S. Prakash, C. J. Brinker, A. J. Hurd and S. M. Rao, Nature, 1995, 374, 439–443 CrossRef CAS.
- S. D. Bhagat, Y. H. Kim, Y. S. Ahn and J. G. Yeo, Microporous Mesoporous Mater., 2006, 96, 237–244 CrossRef CAS PubMed.
- A. V. Rao, A. P. Rao and M. M. Kulkarni, J. Non-Cryst. Solids, 2004, 350, 224–229 CrossRef CAS PubMed.
- P. B. Sarawade, J. K. Kim, A. Hilonga and H. T. Kim, Powder Technol., 2010, 197, 288–294 CrossRef CAS PubMed.
- S. D. Bhagat, K. T. Park, Y. H. Kim, J. S. Kim and J. H. Han, Solid State Sci., 2008, 10, 1113–1116 CrossRef CAS PubMed.
- K. Kanamori, M. Aizawa, K. Nakanishi and T. Hanada, Adv. Mater., 2007, 19, 1589–1593 CrossRef CAS.
- H. B. Chen, Y. Z. Wang and D. A. Schiraldi, ACS Appl. Mater. Interfaces, 2014, 6, 6790–6796 CAS.
- J. Kuhn, T. Gleissner, M. C. Arduini-Schuster, S. Korder and J. Frincke, J. Non-Cryst. Solids, 1995, 186, 291–295 CrossRef CAS.
- M. Liu, L. Gan, Y. Pang, Z. Xu, Z. Hao and L. Chen, Colloids Surf., A, 2008, 317, 490–495 CrossRef CAS PubMed.
- L. Kocon, F. Despetis and J. Phalippou, J. Non-Cryst. Solids, 1998, 225, 96–100 CrossRef CAS.
- T. M. Tillotson and L. W. Hrubesh, J. Non-Cryst. Solids, 1992, 145, 44–50 CrossRef CAS.
- J. C. H. Wong, H. Kaymak, S. Brunner and M. M. Koebel, Microporous Mesoporous Mater., 2014, 183, 23–29 CrossRef CAS PubMed.
- D. Sanli and C. Erkey, ACS Appl. Mater. Interfaces, 2013, 5, 11708–11717 CAS.
- G. M. Pajonk, E. Elaloui, P. Achard, B. Chevalier, J. L. Chevalier and M. Durant, J. Non-Cryst. Solids, 1995, 186, 1–8 CrossRef CAS.
- Y. G. Sun, B. Gates, R. Mayers and Y. N. Xia, Nano Lett., 2002, 2, 165–268 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |