Reversal of regioselectivity in acetylation and deacetylation of aryl-naphthalene diols and diacetates by Amano lipase†
Arpita Panja,
Deb Ranjan Banerjee and
Amit Basak*
Department of Chemistry, Indian Institute of Technology, Kharagpur 721 302, India. E-mail: absk@chem.iitkgp.ernet.in
First published on 25th September 2014
Abstract
The regioselectivity of hydrolysis by Amano lipase (AK lipase) from Pseudomonas fluorescens of aryl naphthalene 4′,7-diacetates 1a–c prepared by the Garratt–Braverman cyclization of bis-propargyl sulfones, ethers and sulfonamides and acetylation of the corresponding diol 2a–c were studied. In all of these cases, the selectivity of both hydrolysis and acetylation were excellent. However, the pattern of selectivity was found to be dependent upon the nature of the fused heterocyclic ring, namely, dihydrothiophene dioxide (sulfolene), dihydrofuran (phthalan) or dihydro isoindole (indoline). For the isoindole system, the hydrolysis as well as the acetylation occurred at C-7, whereas for the furan derivative, the reaction took place entirely at C-4′. Interestingly, for dihydrothiophene dioxide, reversal of selectivity was observed in hydrolysis and acetylation. The results are in contrast with those reported for similar reactions on aryl naphthalene 2′,5-diacetates and corresponding diols. The observed results can be explained by molecular docking using PCL crystal structure, representing the Pseudomonas lipase family.
Introduction
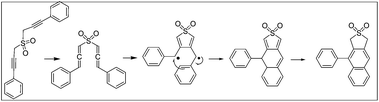
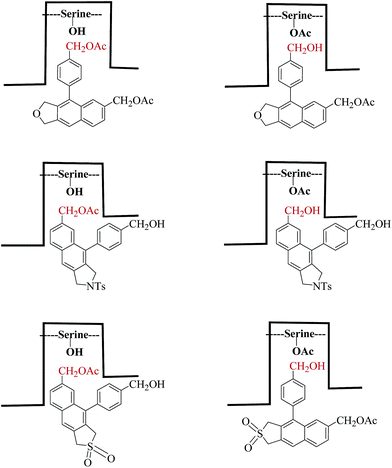
Aryl naphthalene is regarded as a privileged skeleton because of its presence in natural products such as the justicidin class of lignans,1 as well as in several unnatural products with a diverse array of optical and electronic properties.2 Several methods have been reported for the synthesis of such skeletons, which include intramolecular Friedel–Crafts and Diels–Alder reactions.3 In recent years, the use of the Garratt–Braverman (GB) cyclization4 (Scheme 1) for the efficient construction of aryl naphthalenes has been reported along with several mechanistic details.5The usual starting materials for GB cyclization are bis-propargyl sulfones, ethers or sulfonamides. The symmetrical starting materials lead to aryl naphthalenes with the same functional groups at different locations. Unsymmetrical bispropargyl systems with different functionalities at the two ends upon GB cyclization often lead to a mixture of isomers. Thus, there is a need to regiochemically differentiate similar functional groups in arylnaphthalenes obtained via the GB cyclization of symmetrical systems. In a recent paper,6 we reported an enzymatic acetylation/deacetylation route to differentially protected aryl naphthalenes, in which the functional groups were at the C2′ and C5 positions. The binding mode was the same for acetylation and for deacetylation reactions (Scheme 2). We were curious to determine whether similar regioselectivity would be observed for substrates having the acetoxymethyl or hydroxymethyl groups at other positions. Keeping this in mind, we studied the hydrolysis of aryl naphthalene sulfones/ethers/sulfonamide 4′,7-diacetates by Amano lipase (AK lipase)7 from Pseudomonas fluorescens and acetylation of the corresponding diols. In all cases, the reactions are highly regioselective. However, the pattern of selectivity was found to be dependent upon the nature of the fused heterocyclic ring, namely, dihydrothiophene dioxide (sulfolene), dihydrofuran (phthalan) or isoindole (indoline). For the isoindole system, the hydrolysis as well as acetylation occurred at C-7, whereas for the furan derivative, the reactions took place entirely at C4′. For sulfones, the reversal of selectivity for hydrolysis and acetylation was observed. The hydrolysis occurred selectively at C-7, whereas C-4′ was the preferred position for acetylation. The results are in contrast with those reported for similar reactions on 2′,5-disubstituted aryl naphthalenes in which both hydrolysis and acetylation occurred at C-5. The observed results have been explained by molecular docking using the Pseudomonas cepacia lipase crystal structure8 as the representative class of lipases of the Pseudomonas family (Fig. 1).
 | ||
| Fig. 1 Aryl naphthalene diacetates and diols used for enzymatic hydrolysis and acetylation, respectively. | ||
The starting diols 2a–b were prepared by NaBH4 reduction of the corresponding diesters 11a–b,9 whereas the other diol 2c was prepared by deprotection of the THP-protected diol 11c. The diesters and the diol, in turn, were obtained from the bis-propargyl sulfone, ether and sulfonamide by Garratt–Braverman cyclization. The diols were then bis-acetylated with acetyl chloride in the presence of triethylamine (Scheme 3).
The hydrolytic behavior of the aryl naphthalene bis-acetates 1a–c was then studied. Attempted chemical hydrolysis, even under mild conditions (LiOH, THF),11 produced a mixture of monoacetates, thereby demonstrating lack of selectivity. Enzymatic hydrolysis was next attempted using various hydrolytic enzymes. While Candida cylindracea (CCL)12 and porcine pancreatic lipase (PPL)13 failed to induce the hydrolysis of the diacetates or acetylation of the diols, Amano lipase (AK) was able to hydrolyze the diacetates 1a–c to the monoacetates 12a–c in a regioselective manner (Scheme 4). The products of hydrolysis from the diacetates are shown in Table 1.
| Substrate | Product | Enzyme | Yield (%) |
|---|---|---|---|
| 1a | 12a | Amano lipase AK | 75 |
| 1b | 12b | Amano lipase AK | 68 |
| 1c | 12c | Amano lipase AK | 72 |
The acetylation was next carried out using vinyl acetate and Amano lipase (AK), Scheme 5. Again, the reaction proceeded with a high degree of selectivity and the various monoacetates were isolated in high yields (Table 2).
| Substrate | Product | Enzyme | Yield (%) |
|---|---|---|---|
| 2a | 13a | Amano lipase AK | 82 |
| 2b | 13b | Amano lipase AK | 75 |
| 2c | 13c | Amano lipase AK | 75 |
The structure of the various monoacetates obtained upon hydrolysis/esterification could not be confirmed by X-ray crystallography as we failed to obtain good quality crystals. 1H NMR also failed to reveal the location of the acetate. Finally, the structures could be confirmed by studying the shift of aryl hydrogens upon oxidation to the aldehydes, which exerted a deshielding effect on the aryl hydrogens to which the aldehyde functionality is attached. For example, in the aldehyde 14c, which is obtained from 12c, the hydrogens in the pendant aryl ring experienced a downfield shift. For the other aldehyde 15c, which is derived from 13c, the aryl hydrogens in ring A of the naphthalene moiety underwent a downfield shift (Fig. 2).
Inspection of the results shown in Table 1 and 2 revealed many important attributes to these enzyme-catalyzed reactions. Although all the hydrolysis/acetylation reactions were highly regioselective, the pattern of selectivity is dependent upon the nature of heterocyclic ring. Normally, the orientation of substrate binding to the active site of the enzyme for hydrolysis or acetylation is expected to be similar. This is indeed found to be true in case of the furan or isoindole derivative. However, for furan, the substrate binding takes place in such a way that the C4′-acetate or hydroxymethyl is near the active site serine/acetylated serine. For the sulfonamide (1a and 2a), it is the other acetate/hydroxymethyl at C5 that is placed near to the active site serine/acetylated serine upon binding. The hydrolytic/acetylation behaviour of the sulfones is interesting. The orientation of binding for hydrolysis is different from that of acetylation. The hydrolysis takes place preferentially at C5′-acetate, whereas the acetylation has occurred at the C4′-hydroxy methyl. All of these aspects are schematically represented in Scheme 6.
Docking studies: explanation of the results
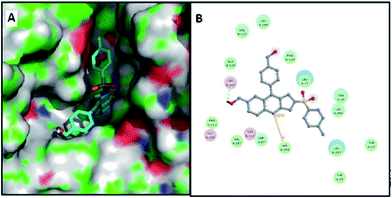
Docking studies have been performed to get an insight into the binding of the presently synthesized compounds. The structure of Pseudomonas fluorescens lipase (PFL) is yet to be determined; therefore, we have performed these docking studies with Pseudomonas cepacia lipase (PCL). PCL is homologous to the PFL and has 22% sequence identity.14 The active site of PCL is constituted by Ser87, His286 and Asp264, whereas the Leu17 and Gln88 residues also take part in the catalysis.15,16 PCL have two binding pockets: one is a large hydrophobic binding pocket, which lies above the active site, i.e. Ser87, and an “alternate binding pocket” lying below the Ser87. This alternate binding pocket is lined with hydrophobic amino acids Tyr23, Leu27, Tyr29, Phe146, Ile290, and Leu293 (Fig. 3). Researchers have shown that “extra hydrogen bonds” with Tyr29 or Tyr 23 residues helped the binding of ligand in this alternate groove and play crucial roles in the selective catalysis.15 Lang et al.17 also suggested that Tyr29 remain hydrogen bonded with active site residues in the native state and during catalysis. Although the crystal structure of PFL is not known, the alignment of the sequence of PFL with PCL suggests a tyrosine (Tyr50) in same position and indicates the presence of an “alternate binding site” in PFL.15 | ||
| Fig. 3 (A) PCL active site (PDB ID: 3LIP) with catalytic triad and other crucial residues; (B) surface representation of PCL, representing the main large binding cavity and alternate binding cavity. | ||
The pattern of selectivity was found to be dependent upon the nature of the fused heterocyclic ring; hence, the differences in their electronic nature, size and position of substituent could have an effect in their binding modes at lipase's active site cavity.
Before proceeding with the docking study, it would be worthy to explore the hydrolysis/acetylation behaviour of some of the representative substrates with PCL and determine whether similar selectivity is followed. Thus, the substrates were treated with lipase, immobilized in sol–gel-AK from Pseudomonas cepacia. Although the reactions were very sluggish, the hydrolysis of diacetate B1 and acetylation of diols C2 and 2a followed similar regioselectivity as revealed by 1H NMR (Fig. 4).
Based on these rationales, we have performed the docking studies. Regio-selective catalysis of similar types of aryl-naphthalenes has been reported previously by this group. As PCL has limited homology with PFL, we have tried to standardize and validate the current in silico approach by using previously reported compounds. The interaction or proximity of ligand parts with active site residue Ser87 has been noted with priority because Ser87 is the most crucial residue and is involved in the first step of the catalysis.
Compounds with fused dihydrofuran ring
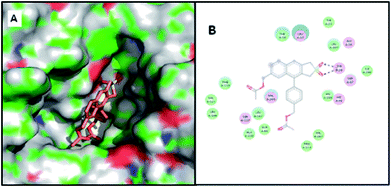
In the case of previously reported compounds, the hydrolysis as well as acetylation occurred at the C-5 position of the naphthalene ring. This previous finding has been supported by the current docking study (details are included in SI†). The substituent at the C-5 position of the naphthalene ring is more exposed than the sterically crowded C-2′ position of the aryl ring. In the case of both the hydrolysis and acetylation reactions, the corresponding compound was docked at main binding cavity. The acetate/alcohol linked to C-5 has been found to interact with crucial residues line Tyr29/Ser117 and remain in close proximity to the Ser87/acylated-Ser87. The interactions of the substituent with lipase have been found to be the major driving force behind region-selectivity of this class of compounds.In the case of current compounds, the hydrolysis as well as acetylation occurred at the C-4′ position of the aryl ring. The substituent linked to the C-4′ position of the aryl ring is more exposed than the C-2′ position of the previous cases. Docking studies indicate that the naphthalene ring docked at main binding cavity, whereas the acetate/alcohol linked to C-4′ dragged the aryl ring towards active site via interacting with crucial residues like Tyr29 and Leu17 (Fig. 5 and 6).
Compounds with fused dihydroisoindole ring
In the case of previously reported compounds, the hydrolysis as well as acetylation occurred at the C-5 position of the naphthalene ring. We have studied these previous findings as model studies. The docking study suggests that the region-selectivity of these classes of compounds has been guided by the positioning of the N-tosyl group at the binding cavity of lipase. The large and hydrophobic N-tosyl group always acquires the “alternate binding cavity” and forces the naphthalene ring pointing towards the active-site cavity. Hence, the reaction takes place at the C-5 position (details are included in SI†).In the case of current compounds, the hydrolysis as well as acetylation occurred at the C-7 position of the naphthalene ring. From the docking study, we have found similar results similar to the model study. The bulky and hydrophobic N-tosyl unit position in an alternate binding cavity forces the naphthalene ring to point towards active site cavity. Hence, reactions take place at the C-7 position regio-selectively (Fig. 7 and 8).
Compounds with dihydrothiophene dioxide ring
The docking study of this class of compounds is intriguing, and it suggests that both the sulfone group and substituent present at the ligand molecule can cause sufficient interaction with the lipase receptor, which can govern the regioselectivity. In the case of previously reported compounds, the hydrolysis as well as acetylation occurred at the C-5 position of the naphthalene ring. However, the docking study predicts that the reactions should occur at the C-2′ position (details are included in SI†). We obtained the same trend of regio-selectivity with alternate positioning, which may be due to the fact that we have performed this computational study with PCL, and we have conducted experiments with PFL. The sulfone group itself can cause strong interaction with protein residues unlike furan/tosyl; hence, small changes in sequences or cavity sizes between PCL and PFL can trigger the position alternation. If we take this result as a model study, we should then obtain similar results in the case of current sulfones as well.In the case of current compounds, the reversal of selectivity for hydrolysis and acetylation was observed experimentally. The docking study supports this reversal of selectivity with alternate positioning similar to our model study. The hydrolysis reaction takes place at the C-7 position, whereas the docking study predicts that the reaction will take place at the C-4′ position. The sulfone group strongly interacts with Tyr29 and drags the aryl ring towards the active site cavity (Fig. 9). The acetylation reaction takes place at the C-4′ position, whereas the docking study suggests the reaction takes place at the C-7 position. In the acetylation process, the sulfone group does not interact with lipase, but the alcohol group at the C-7 position interacts directly with acylated Ser87 residue and governs the reaction (Fig. 10).
From the above mentioned exercise, it is clear that the docking can successfully explain the observed regioselectivity in the case of the isoindole and furan derivatives. While the interaction of the substituent with lipase has been the major driving force for furans, the positioning of the bulky N-tosyl group drives the selectivity in the case of isoindole derivatives. The docking results of sulfone derivatives are more intriguing. The study has suggested that the combined interactions of the sulfone group and the substituent may be the probable reason behind the reversal of selectivity for hydrolysis and acetylation. For hydrolysis, the polar sulfone is the main binding force unlike furan/tosyl, whereas for acetylation, the free alcohol group is involved in the major interaction. Although docking studies could not explain the position alternation, it is likely that the sulfone group itself can cause strong interaction with protein residues. A small change between PCL and PFL structures can cause the position alternation. This was observed in the acetylation of diol 2a catalysed by AK lipase and PCL (Fig. 11).
 | ||
| Fig. 11 Comparative 1H NMR of PFL- and PCL-catalyzed acetylations. (a) Starting diol; (b) and (d) regio-isomeric monoacetate from PFL-catalyzed acetylation; (c) PCL-catalyzed reaction acetylation. | ||
In conclusion, we have shown that differently functionalized aryl naphthalenes can be prepared by Amino lipase (AK)-catalyzed hydrolysis or acetylation of the corresponding diacetates or diols, respectively. It has also been demonstrated that a change in the fused heterocycle can affect the nature of binding to the active site. Future work will address the issue of axial chirality during the enzyme-catalyzed hydrolysis/acetylation of substrates capable of showing atropisomerism.
Experimental section
Docking details
Advanced and widely used molecular grid-based docking program Autodock4.2 was used to predict the binding modes and approximate binding free energies of all the designed inhibitors in lead library. The X-ray crystal coordinates of PCL was obtained from the Protein Data Bank (http://www.rcsb.org), PDB ID 3LIP. Receptor structure had been edited and hydrogen atoms added prior to docking. AutoDock tools was used to assign Gasteiger charges to the receptor. Ligand structures were built up using Accelrys Discovery studio 3.1 client. Energy minimization of ligand structures was performed by applying CHARMM force field. The conformer with best binding energy was evaluated for designing purpose.Chemistry details
All reactions were conducted with oven-dried glassware under nitrogen. All common reagents were commercial grade reagents and used without further purification. The solvents were dried by standard methods and purified by distillation before use. Silica gel (60–120 and 230–400 mesh) was used for column chromatography. TLC was performed on aluminum-backed plates coated with silica gel 60 with F254 indicator. Locally available UV-lamp chamber and I2-blower were used as the TLC spot indicator. For solid compounds, melting point (MP) was measured in melting point apparatus twice and reported without further calibration. The NMR spectra were recorded using 200 MHz and 400 MHz. The following abbreviations are used to describe peak patterns: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = double doublet, ABq = AB quartet.Synthesis of 11a. To the solution of bis-propargylsulfone, 10a (1.4 mmol) in dry CHCl3, Et3N (2.8 mmol) was added and stirred for 1 h at room temperature. The reaction mixture was partitioned between water–dichloromethane. The organic layer was washed with brine and dried over Na2SO4. After evaporation of the solvent, the crude product was purified by column chromatography over Si gel using petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (2
ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. State: sticky mass; yield: 92%; δH (400 MHz, CDCl3) 8.28–8.24 (3H, m), 8.14 (1H, d, J = 8.8 Hz), 7.95 (2H, d, J = 8.4 Hz), 7.43 (2H, d, J = 8 4 Hz), 4.63 (2H, s), 4.25 (2H, s), 4.02 (3H, s), 3.89 (3H, s); δC (100 MHz, CDCl3) 166.7, 166.5, 137.3, 137.0, 134.0, 132.4, 131.3, 131.0, 130.7, 130.5, 129.9, 129.7, 129.4, 128.6, 126.7, 126.6, 126.4, 57.0, 56.4, 52.5.
1) as eluent. State: sticky mass; yield: 92%; δH (400 MHz, CDCl3) 8.28–8.24 (3H, m), 8.14 (1H, d, J = 8.8 Hz), 7.95 (2H, d, J = 8.4 Hz), 7.43 (2H, d, J = 8 4 Hz), 4.63 (2H, s), 4.25 (2H, s), 4.02 (3H, s), 3.89 (3H, s); δC (100 MHz, CDCl3) 166.7, 166.5, 137.3, 137.0, 134.0, 132.4, 131.3, 131.0, 130.7, 130.5, 129.9, 129.7, 129.4, 128.6, 126.7, 126.6, 126.4, 57.0, 56.4, 52.5.
Synthesis of 11b. DBU (1.4 mmol) was added to the solution of bis-propargyl tosyl amine, 10b (0.7 mmol) in dry toluene, and refluxed for 48 h. After completion of the reaction, toluene was removed under vacuo. The crude product was extracted with ethyl acetate and after removing the solvent, it was purified by column chromatography using petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (3
ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. State: sticky mass; yield: 90%; δH (400 MHz, CDCl3) 8.25–8.23 (3H, m), 8.07 (1H, d, J = 8.4 Hz), 7.89 (1H, d, J = 8.4 Hz), 7.77–7.73 (3H, m), 7.38 (2H, d, J = 8.4 Hz), 7.33 (2H, d, J = 8.4 Hz), 4.83 (2H, s), 4.47 (2H, s), 4.03 (3H, s), 3.88 (3H, s), 2.41 (3H, s); δC (100 MHz, CDCl3) 167.0, 166.8, 144.0, 141.6, 137.0, 135.7, 134.8, 134.5, 133.3, 130.7, 130.3, 130.2, 130.0, 129.6, 128.4, 128.3, 128.0, 127.7, 125.7, 121.2, 53.6, 52.9, 52.5, 52.4, 21.6.
1) as eluent. State: sticky mass; yield: 90%; δH (400 MHz, CDCl3) 8.25–8.23 (3H, m), 8.07 (1H, d, J = 8.4 Hz), 7.89 (1H, d, J = 8.4 Hz), 7.77–7.73 (3H, m), 7.38 (2H, d, J = 8.4 Hz), 7.33 (2H, d, J = 8.4 Hz), 4.83 (2H, s), 4.47 (2H, s), 4.03 (3H, s), 3.88 (3H, s), 2.41 (3H, s); δC (100 MHz, CDCl3) 167.0, 166.8, 144.0, 141.6, 137.0, 135.7, 134.8, 134.5, 133.3, 130.7, 130.3, 130.2, 130.0, 129.6, 128.4, 128.3, 128.0, 127.7, 125.7, 121.2, 53.6, 52.9, 52.5, 52.4, 21.6.
Synthesis of 11c. To the solution of bis-propargyl ether (0.6 mmol) in toluene, potassium t-butoxide (1.2 mmol) was added and was allowed to reflux for 6 h. After completion of the reaction, toluene was removed under vacuo. The reaction mixture was partitioned between water and ethyl acetate. The organic layer was washed with brine and dried over Na2SO4. The crude product was purified by column chromatography over Si gel using petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (3
ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. State: gummy liquid; yield: 90%; δH (400 MHz, CDCl3) 7.88 (1H, d, J = 8.4 Hz), 7.69 (2H, s), 7.53 (3H, d, J = 8.1 Hz), 7.37 (2H, d, J = 6.6 Hz), 5.30 (2H, s), 5.05 (2H, s), 4.94 (1H, d, J = 12 Hz), 4.88–4.84 (2H, m), 4.72–4.70 (1H, m), 4.64–4.57 (2H, m), 3.99–3.89 (2H, m), 3.66–3.52 (2H, m), 1.68–1.52 (12H, m); δC (100 MHz, CDCl3) 138.0, 137.8, 137.4, 137.3, 136.0, 133.4, 132.6, 131.9, 129.7, 128.5, 128.1, 126.0, 124.6, 118.8, 98.3, 98.0, 73.6, 73.2, 69.2, 68.9, 62.4, 30.8, 25.7, 20.0, 19.6.
1) as eluent. State: gummy liquid; yield: 90%; δH (400 MHz, CDCl3) 7.88 (1H, d, J = 8.4 Hz), 7.69 (2H, s), 7.53 (3H, d, J = 8.1 Hz), 7.37 (2H, d, J = 6.6 Hz), 5.30 (2H, s), 5.05 (2H, s), 4.94 (1H, d, J = 12 Hz), 4.88–4.84 (2H, m), 4.72–4.70 (1H, m), 4.64–4.57 (2H, m), 3.99–3.89 (2H, m), 3.66–3.52 (2H, m), 1.68–1.52 (12H, m); δC (100 MHz, CDCl3) 138.0, 137.8, 137.4, 137.3, 136.0, 133.4, 132.6, 131.9, 129.7, 128.5, 128.1, 126.0, 124.6, 118.8, 98.3, 98.0, 73.6, 73.2, 69.2, 68.9, 62.4, 30.8, 25.7, 20.0, 19.6.
Compound 2a. State: white solid; yield: 90%; mp: 200 °C; δH (400 MHz, CDCl3) 7.88 (1H, d, J = 8.5 Hz), 7.85 (1H, s), 7.59–7.52 (4H, m), 7.29 (2H, d, J = 8.0 Hz), 4.83 (2H, s), 4.73 (2H, s), 4.59 (2H, s), 4.22 (2H, s); δC (100 MHz, CDCl3) 141.2, 139.8, 138.0, 136.9, 133.0, 132.3, 130.0, 128.7, 128.7, 128.6, 127.8, 127.7, 126.5, 125.0, 123.9, 65.5, 65.2.
Compound 2b. State: white solid; yield: 90%; mp: 220 °C; δH (400 MHz, CDCl3) 7.81 (1H, d, J = 8.4 Hz), 7.73 (2H, d, J = 8.0 Hz), 7.63 (1H, s), 7.52–7.47 (4H, m), 7.31–7.24 (4H, m), 4.83 (2H, s), 4.78 (2H, s), 4.69 (2H, s), 4.45 (2H, s), 2.39 (3H, s); δC (100 MHz, CDCl3) 143.7, 140.5, 138.6, 136.7, 134.3, 134.0, 133.8, 133.0, 131.7, 129.8, 129.5, 128.4, 127.6, 127.4, 125.4, 123.3, 120.5, 65.4, 65.0, 53.5, 53.1, 22.4.
Procedure for the synthesis of 2c. To the solution of 11c in ethanol PPTS and drop of water were added and allowed to stir at 50 °C for 8 h. Ethanol was removed under vacuo. The mixture was partitioned between water–ethyl acetate. The organic layer was washed with brine and dried over Na2SO4. After removing the solvent, the pure product was obtained by column chromatography over Si gel, petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (1
ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent.
2) as eluent.State: sticky mass; yield: 85%; δH (400 MHz, CDCl3) 7.89 (1H, d, J = 8.4 Hz), 7.71 (1H, s), 7.63 (1H, s), 7.51 (3H, d, J = 8.0 Hz), 7.34 (2H, d, J = 7.6 Hz), 5.31 (2H, s), 5.0 (2H, s), 4.81 (2H, s), 4.73 (2H, s); δC (100 MHz, CDCl3) 140.5, 138.5, 137.8, 137.5, 137.4, 133.4, 132.5, 131.9, 129.8, 128.8, 127.5, 125.4, 123.5, 118.9, 73.5, 73.1, 65.7, 65.3.
Compound 1a. State: sticky yellow mass; yield: 80%; δH (400 MHz, CDCl3) 7.90 (1H, d, J = 8.4 Hz), 7.85 (1H, s), 7.57–7.54 (3H, m), 7.50 (1H, s), 7.33–7.29 (2H, m), 5.25 (2H, s), 5.15 (2H, s), 4.61 (2H, s), 4.25 (2H, s), 2.20 (3H, s), 2.08 (3H, s); δC (100 MHz, CDCl3) 171.0, 170.8, 137.7, 137.2, 136.4, 134.8, 133.0, 131.9, 129.8, 129.1, 128.9, 128.6, 127.0, 125.6, 124.9, 66.3, 65.9, 57.2, 56.5, 21.2, 21.0.
Compound 1b. State: sticky mass; yield: 70%; δH (400 MHz, CDCl3) 7.84 (1H, d, J = 8.4 Hz), 7.77 (2H, d, J = 8.0 Hz), 7.66 (1H, s), 7.55–7.46 (4H, m), 7.33 (2H, d, J = 7.6 Hz), 7.28 (2H, d, J = 7.6 Hz), 5.26 (2H, s), 5.13 (2H, s), 4.81 (2H, s), 4.49 (2H, s), 2.42 (3H, s), 2.23 (3H, s), 2.06 (3H, s); δC (100 MHz, CDCl3) 171.2, 171.0, 144.0, 137.4, 136.0, 135.0, 134.3, 134.1, 133.9, 133.6, 133.4, 131.7, 130.1, 129.8, 129.0, 128.7, 127.9, 126.3, 125.3, 120.9, 66.6, 66.2, 53.7, 53.3, 21.7, 21.3, 21.2.
Compound 1c. State: gummy liquid; yield: 70%; δH (400 MHz, CDCl3) 7.87 (1H, d, J = 8.4 Hz), 7.69 (1H, s), 7.61 (1H, s), 7.51–7.46 (3H, m), 7.35 (2H, d, J = 8.0 Hz), 5.28 (2H, s), 5.22 (2H, s), 5.14 (2H, s), 5.01 (2H, s), 2.18 (3H, s), 2.06 (3H, s); δC (100 MHz, CDCl3) 171.1, 171.0, 138.3, 137.9, 137.6, 135.6, 133.5, 133.5, 132.3, 131.6, 129.8, 129.0, 128.8, 128.7, 126.0, 125.3, 119.0, 73.5, 73.0, 66.6, 66.2, 21.2, 21.1.
General procedure for hydrolysis of the diacetate. To the solution of diacetates 1a–c (0.14 mmol) in acetone–phosphate buffer (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v, pH 7), the enzyme Amano lipase (18 mg) was added and stirred at room temperature for 6 h. The reaction mixture was filtered and acetone was removed under vacuo. The crude was extracted with ethyl acetate, and the organic layer was washed with brine and dried over Na2SO4. After removal of the solvent, the crude was purified by column chromatography over Si gel using petroleum ether and ethyl acetate (2
3 v/v, pH 7), the enzyme Amano lipase (18 mg) was added and stirred at room temperature for 6 h. The reaction mixture was filtered and acetone was removed under vacuo. The crude was extracted with ethyl acetate, and the organic layer was washed with brine and dried over Na2SO4. After removal of the solvent, the crude was purified by column chromatography over Si gel using petroleum ether and ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to get the pure monoacetates 12a–c.
1) as eluent to get the pure monoacetates 12a–c.State: sticky mass; yield: 75%; δH (400 MHz, CDCl3) 7.88 (1H, d, J = 8.4 Hz), 7.83 (1H, s), 7.58–7.51 (4H, m), 7.31–7.29 (2H, m), 5.24 (2H, s), 4.75 (2H, s), 4.59 (2H, s), 4.23 (2H, s), 2.19 (3H, s); δC (100 MHz, CDCl3) 171.1, 139.8, 137.6, 137.4, 136.3, 132.8, 132.0, 129.8, 128.9, 128.6, 128.5, 128.4, 127.6, 126.4, 124.9, 123.7, 65.9, 65.3, 57.2, 56.6, 21.2.
State: semi solid; yield: 68%; δH (400 MHz, CDCl3) 7.82 (1H, d, J = 8.9 Hz), 7.74 (2H, d, J = 8.0 Hz), 7.64 (1H, s), 7.50 (4H, d, J = 9.4 Hz), 7.31 (4H, d, J = 8.1 Hz), 5.23 (2H, s), 4.78 (2H, s), 4.71 (2H, s), 4.46 (2H, s), 2.39 (3H, s), 2.21 (3H, s); δC (100 MHz, CDCl3) 171.2, 144.0, 138.9, 137.6, 135.9, 134.5, 133.5, 134.1, 133.2, 131.8, 130.1, 129.8, 129.0, 128.7, 127.9, 125.7, 123.5, 120.9, 66.2, 65.7, 53.7, 53.3, 21.8, 21.4. State: sticky mass; yield: 72%; δH (400 MHz, CDCl3) 7.88 (1H, d, J = 8.4 Hz), 7.69 (1H, s), 7.62 (1H, s), 7.52 (2H, d, J = 7.8 Hz), 7.47 (1H, d, J = 8.4 Hz), 7.35 (2H, d, J = 7.7 Hz), 5.29 (2H, s), 5.14 (2H, s), 5.01 (2H, s), 4.83 (2H, s), 2.06 (3H, s); δC (100 MHz, CDCl3) 171.1, 140.5, 138.4, 137.6, 137.4, 133.5, 132.6, 131.8, 129.9, 128.9, 127.6, 126.0, 125.6, 124.7, 124.2, 119.3, 118.9, 73.6, 73.1, 66.9, 65.4, 21.2.
General procedure for the acetylation of the diol. Vinyl acetate (0.68 mmol) and Amano lipase (44 mg) were added to the THF solution of diol 2a–c (0.34 mmol). The reaction mixture was allowed to stir at room temperature until the reaction was complete (6 h for 2a; 4 h for 2b and 2c). It was then filtered and THF was removed under vacuo. The pure monoacetates 12a and 13b–c were isolated by column chromatography of the crude residue over Si gel using petroleum ether and ethyl acetate (2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent.
1) as eluent.State: sticky mass; yield: 82%; δH (400 MHz, CDCl3) 7.87 (1H, d, J = 8.4 Hz), 7.81 (1H, s), 7.56–7.49 (4H, m), 7.29–7.28 (2H, m) 5.22 (2H, s), 4.73 (2H, s), 4.58 (2H, s), 4.21 (2H, s), 2.18 (3H, s); δC (100 MHz, CDCl3) 171.2, 139.9, 137.7, 137.5, 136.4, 132.9, 132.2, 129.9, 129.0, 128.7, 128.6, 128.5, 126.5, 125.1, 123.8, 66.1, 65.4, 57.3, 56.7, 21.3.
State: semi solid; yield: 75%; δH (400 MHz, CDCl3) 7.81 (1H, d, J = 9.6 Hz), 7.73 (2H, d, J = 8.0 Hz), 7.63 (1H, s), 7.53–7.49 (3H, m), 7.43 (1H, d, J = 8.0 Hz), 7.29 (3H, d, J = 8.0 Hz), 7.23 (1H, s), 5.09 (2H, s), 4.83 (2H, s), 4.78 (2H, s), 4.46 (2H, s), 2.39 (3H, s), 2.04 (3H, s); δC (100 MHz, CDCl3) 170.8, 143.7, 140.6, 136.4, 134.7, 134.1, 134.0, 133.6, 133.4, 133.1, 131.5, 129.8, 129.5, 128.4, 127.6, 127.4, 126.0, 125.2, 120.5, 65.0, 64.4, 53.5, 53.0, 21.5, 20.9.
State: sticky mass; yield: 75%; δH (400 MHz, CDCl3) 7.86 (1H, d, J = 8.4 Hz), 7.68 (1H, s), 7.60 (1H, s), 7.50–7.47 (3H, m), 7.35–7.33 (2H, m), 5.27 (2H, s), 5.21 (2H, s), 4.99 (2H, s), 4.73 (2H, s), 2.17 (3H, s); δC (100 MHz, CDCl3) 171.0, 138.5, 138.0, 137.7, 137.3, 135.4, 133.2, 132.1, 131.7, 129.7, 128.6, 128.5, 125.2, 123.3, 118.8, 73.3, 72.9, 66.0, 65.5, 21.1.
Acknowledgements
The author AB is grateful to DST, Government of India, for research funding and the JC Bose fellowship. AP and DB thank CSIR, Government of India, for a research fellowship (NET). DST is also acknowledged for providing the funds for a 400 MHz NMR facility under the IRPHA programme.References
- (a) Y. H. Hui, C. J. Chang, J. L. Mclaughlin and R. G. Powell, J. Nat. Prod., 1986, 49, 1175 CrossRef CAS; (b) C. C. Chen, W. C. Hsin, N. F. Ko, Y. L. Huang, C. J. Ou and C. M. Teng, J. Nat. Prod., 1996, 59, 1149 CrossRef CAS PubMed; (c) A. Mohagheghzadeh, T. J. Schmidt and A. W. Alfermann, J. Nat. Prod., 2002, 65, 69 CrossRef CAS PubMed; (d) N. Vasile, Elfahmi, R. Boss, O. Kayser, G. Momekov, S. Konstantinov and I. Ionkova, J. Nat. Prod., 2006, 69, 1014 CrossRef PubMed.
- (a) D. S. Tyson, A. D. Carbaugh, F. Ilhan, J. Santos-Perez and M. A. Meader, Chem. Mater., 2008, 20, 6595 CrossRef CAS; (b) M. A. Meader, D. S. Tyson and F. Ilhan, US 20080242870 A1 20081002, 2008; (c) M. A. Meader, D. S. Tyson and A. D. Carbaugh, PMSE Prepr., 2008, 98, 130 Search PubMed; (d) A. Facchetti, T. J. Marks and H. Yan, WO.2008085942 A2 20080717, 2008.
- (a) S. Kobayashi, Cycloaddition Reactions in Organic Synthesis, Wiley-VCH, 2001 CrossRef; (b) W. Carruthers, Cycloaddition Reactions in Organic Synthesis, Pergamon Press, Oxford, 1990 Search PubMed.
- (a) S. Braverman and D. Segev, J. Am. Chem. Soc., 1974, 96, 1245 CrossRef CAS; (b) P. J. Garratt and S. B. Neoh, J. Org. Chem., 1979, 44, 2667 CrossRef CAS; (c) Y. S. P. Cheng, P. J. Garratt, S. B. Neoh and V. H. Rumjanek, Isr. J. Chem., 1985, 26, 101 CrossRef CAS; (d) S. Braverman, Y. Duar and D. Segev, Tetrahedron Lett., 1976, 17, 3181 CrossRef; (e) Y. Zafrani, H. E. Gottlieb, M. Sprecher and S. Braverman, J. Org. Chem., 2005, 70, 10166 CrossRef CAS PubMed.
- (a) M. Maji, D. Mallick, S. Mondal, A. Anoop, S. S. Bag, A. Basak and E. D. Jemmis, Org. Lett., 2011, 13, 888 CrossRef CAS PubMed.
- A. Panja, D. Ghosh and A. Basak, Bioorg. Med. Chem. Lett., 2013, 23, 893 CrossRef CAS PubMed.
- R. Gupta, N. Gupta and P. Rathi, Appl. Microbiol. Biotechnol., 2004, 64, 763 CrossRef CAS PubMed.
- K. K. Kim, K. Y. Song, D. H. Shin, K. Y. Hwang and S. W. Suh, Structure, 1997, 5, 173 CrossRef CAS.
- (a) A. Saeed and Z. Ashraf, J. Chem. Sci., 2006, 118, 419 CrossRef CAS; (b) J. D. Prugh and A. A. Deana, Tetrahedron Lett., 1988, 29, 37 CrossRef CAS.
- (a) S. Mandal, M. Maji and A. Basak, Tetrahedron Lett., 2011, 52, 1183 CrossRef PubMed; (b) R. Mukherjee, S. Mandal, A. Basak, D. Mallick and E. D. Jemmis, Chem.–Asian J., 2012, 7, 957 CrossRef CAS PubMed; (c) R. Mukherjee and A. Basak, Synlett, 2012, 877 CAS; (d) S. Mondal, R. Mukherjee, A. Anoop and A. Basak, Tetrahedron, 2012, 68, 7202 CrossRef CAS PubMed.
- (a) D. A. Evans, S. J. Miller, M. D. Ennies and P. L. Ornstein, J. Org. Chem., 1992, 57, 1067 CrossRef CAS; (b) A. B. Smith, S. S.-Y. Chen, F. C. Nelsen, J. M. Reichert and B. A. Salvatore, J. Am. Chem. Soc., 1997, 119, 10953 Search PubMed; (c) M. Nazare and H. Waldman, Chem.–Eur. J., 2001, 7, 3363 CrossRef CAS.
- (a) R. Sharma, Y. Chisti and U. C. Banerjee, Biotechnol. Adv., 2001, 19, 627 CrossRef CAS; (b) Y. Y. Linko, M. Lamsa, A. Huhtala and O. Rantanen, J. Am. Oil Chem. Soc., 1995, 72, 129 CrossRef.
- A. Zaks and A. M. Klibanov, Science, 1984, 224, 1291 Search PubMed.
- Y. Tan and K. J. Miller, Cloning, expression, and nucleotide sequence of a lipase gene from Pseudomonas fluorescens; B52, Appl. Environ. Microbiol., 1992, 58, 1402 CAS.
- W. V. Tuomi and R. J. Kazlauskas, J. Org. Chem., 1999, 64, 2638 CrossRef CAS PubMed.
- (a) R. P. Hof and R. M. Kellogg, J. Chem. Soc., Perkin Trans. 1, 1996, 2051 RSC; (b) K. Lemke, M. Lemke and F. Theil, J. Org. Chem., 1997, 62, 6268 CrossRef CAS.
- D. Lang, B. Hofmann, L. Haalck, H. J. Hecht, F. Spener, R. D. Schmid and D. Schomburg, J. Mol. Biol., 1996, 259, 704 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10503f |
| This journal is © The Royal Society of Chemistry 2014 |