Reduced graphite oxide/SnO2/Au hybrid nanomaterials for NO2 sensing performance at relatively low operating temperature
Hao Zhang,
LiLi Wang and
Tong Zhang*
State Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, PR China. E-mail: zhangtong@jlu.edu.cn; Fax: +86 431 85168270; Tel: +86 431 85168385
First published on 22nd October 2014
Abstract
The reduced graphene oxide/SnO2/Au (rGO/SnO2/Au) hybrid nanomaterials have been prepared by a one-step hydrothermal process with a property of gas sensing at a relatively low operating temperature. The structure and morphology characteristics of the resultant product were investigated by X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and high-resolution transmission electron microscopy (HRTEM). The sizes of the Au nanoparticles of rGO/SnO2/Au-1 to rGO/SnO2/Au-4 range from about 12 to 90 nm, respectively. To demonstrate the usage of such hybrid nanomaterials, chemical gas sensors have been fabricated and investigated for NO2 detection. It is found that the rGO/SnO2/Au sensor exhibits much better response/recovery time which is 19/20 s at the optimal operating temperature of 50 °C to 5 ppm NO2, compared with the pure rGO (798/8319 s) and rGO/SnO2 (427/908 s). The enhanced sensing features can be attributed to the heterojunctions with the highly conductive graphene, SnO2 thin film and Au nanoparticles.
1. Introduction
NO2 as a typical air pollutant mainly comes from the exhaust gases of combustion processes, which severely affects the respiratory system of human beings and animals.1,2 Large amounts of NO2 have been released to the environment by combustion system and vehicle every year. Therefore, the detection of NO2 is imperatively necessary for industrial and domestic appliances. Numerous techniques have been developed to detect trace levels of NO2, such as chemiluminescence,3 ion chromatography,4 spectrophotometry,5 etc. However, with the growing awareness about environmental pollution and occupational safety issues over the recent decades, how to make high performance sensors for NO2 has become an important subject.Graphene, known as “the thinnest material in our universe” with only one-atom thickness,6 has attracted huge attention for its high electron mobility since discovery.7–9 Owing to the 2D structure, graphene can adsorb highly sensitive molecules which treat every carbon atom as a surface atom. Because of its unique features of high surface area, light weight, high electron mobility and mechanical strength, graphene can make a highly promising range of applications such as supercapacitors, nanoelectronics, sensors, hydrogen storage and so forth.6–13 Graphene oxide (GO) can form well-dispersed aqueous colloids without stabilizers,14–16 during to electrostatic repulsion of the versatile oxygen-containing groups (OCGs). Also it is of particular interest that the aqueous colloidal dispersion of GO can be used as the starting support material for fabricating advanced graphene-based nanomaterials.17 Recently, the synthesis of hybrid nanomaterials based on graphene has flashed enormous research interest.10,17–20 Compared with the graphene, reduced graphene oxide is expected to be a promising sensing materials due to its semiconductor properties.21–25 To promote the sensitivity and particularly the selectivity of the rGO based sensors, rGO decorated with noble metals and metal oxides have been proposed.26 Chen et al. recently reported a room temperature NO2 gas sensor using a Co3O4 doped-graphene film.27
In this paper, we have fabricated rGO/SnO2 nanocomposites decorated with Au nanoparticles in high concentration of a GO precursor which was achieved by introducing a certain amount of HAuCl4 and SnCl2 into the reaction system. The structural and morphology characteristics have been characterized. The resulting hybrid nanomaterials have been used as gas sensors, which exhibit good performance to NO2 at operating temperature (50 °C). The effect of the ratio of Au on the sensitivity is investigated. The influences of different operating temperature and gas concentration on the sensitive characteristics have also been investigated. In addition, the sensing mechanism for the detection of NO2 is also discussed.
2. Experimental
2.1. Chemicals
Graphite powder, H2O2 (30%), NaNO3, H2SO4 (98%), SnCl2, KMnO4 and HAuCl4 were purchased from Beijing Chemical Corp. All chemicals were used as received without further purification.2.2. Preparation of GO
GO was prepared from natural graphite powder through a modified Hummers’ method.28 In a typical synthesis, 1 g of graphite was added into 23 mL of H2SO4, followed by stirring at room temperature for 24 h. After that, 100 mg of NaNO3 was introduced into the mixture and stirred for 30 min. Subsequently, the mixture was kept below 5 °C by ice bath, and 3 g of KMnO4 was slowly added into the mixture. After being heated to 35–40 °C, the mixture was stirred for another 30 min. After that, 46 mL of water was added into the above mixture during a period of 25 min and the mixture was heated to 95 °C under stirring for 15 min. Finally, 140 mL of water and 10 mL of H2O2 were added into the mixture to stop the reaction. After the unexploited graphite in the resulting mixture was removed by centrifugation, as-synthesized GO was dispersed into individual sheets in distilled water at a concentration of 1 mg mL−1 with the aid of ultrasound for further use.2.3. Preparation of rGO/SnO2/Au nanocomposites
The rGO/SnO2/Au nanocomposites were prepared according to previous report.29 In a typical synthesis, 0.5 ml of GO (1 mg mL−1) were added into 20 ml of water, followed by stirring for 10 min to get a homogeneous yellow-brown colloidal. Then 0.024 g of SnCl2 and HCl (38%) was introduced into the GO solution, which was sonicated for 40 min, followed by addition of HAuCl4 (0.01 M). After further sonicating for 30 min, the aqueous dispersion was transferred into a 40 mL Teflon-lined, stainless-steel autoclave and heated at 180 °C for 12 h. The black product was harvested by centrifugation and washed with water and ethanol several times, and dried at 60 °C for several hours. Four different rGO/SnO2/rGO samples with the Au ratio of 0.5%, 1%, 2% and 5% by weight were obtained, designated as rGO/SnO2/Au-1, rGO/SnO2/Au-2, rGO/SnO2/Au-3 and rGO/SnO2/Au-4, respectively. Similarly, the rGO and rGO/SnO2 were also prepared.2.4. Characterizations
The composition and phase of the products were characterized by X-ray powder diffraction (XRD, Rigaku D/max-2500) with graphite monochromatized and Cu Kα λ = 0.15418 nm and X-ray photoelectron spectroscopy (XPS), and Raman spectroscopy, respectively. The structure and morphology of the samples were obtained by transmission electron microscopy (TEM, JEOL JEM-3010).2.5. Fabrication and measurement of gas sensors
The prepared rGO/SnO2/Au nanocomposites were mixed with deionized water to obtain a solution in a mortar. Fig. 1a and b shows a schematic image and photographs of this sensor substrate. The droplet has covered a ceramic plate (1 mm × 1.5 mm), which was previously covered with gold electrodes and ruthenium oxides as heater on front and back sides by screen printing technique, followed by drying at room temperature. Fig. 1c shows the schematic diagram of the test circuit. The operating temperature of the rGO/SnO2/Au sensors was adjusted by varying the heating voltage (VH). By monitoring VS, the resistance of the sensor in air or a test gas can be measured.30 The response of the sensor was defined as the ratio of the sensor resistance in dry air (Ra) to that in target gas (Rg), which is measured between 30 and 60 °C. The time taken by the sensor to achieve 90% of the total resistance change was defined as the response time in the case of adsorption or the recovery time in the case of desorption.31 The gas-sensing properties of the sensors were measured using a CGS-8 gas-sensing characterization system.3. Results and discussions
3.1. Structural and morphological characteristics
The phase composition of the hybrid structures is analyzed by XRD. Fig. 2a shows the XRD patterns of GO. It is seen that a strong peak of 2θ at 11.26° corresponding to the (002) interlayer d spacing of 7.85 Å was observed, indicating the successful preparation of GO by oxidation of graphite.32 However, no diffraction peaks can be ascribed to graphene in Fig. 2a, probably due to self-reassembling of exfoliated graphite oxide or the reduction of GO by hydrothermal treatment.33 Fig. 2b–f demonstrate the XRD patterns of rGO/SnO2 and rGO/SnO2/Au and show four highly broadened peaks, 26.56, 33.82, 51.50 and 65.42°, which are attributed to the (100), (101), (211) and (112) planes of tetragonal rutile SnO2 (JCPDS Card no. 41-1049),34 indicating the formation of SnO2 crystals. Fig. 2g shows the EDX pattern of the rGO/SnO2/Au-2 sample. EDX analysis shows the rGO/SnO2/Au-2 sample was composed of C, Sn, O and Au element. Weak peaks for Au could be seen in Fig. 2g. This may be due to the low content of Au nanoparticles in the composite materials.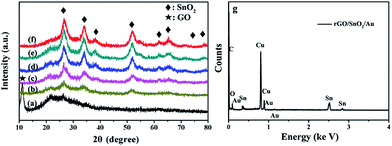 | ||
| Fig. 2 The XRD patterns of the products: (a) GO, (b) rGO/SnO2, (c) rGO/SnO2/Au-1, (d) rGO/SnO2/Au-2, (e) rGO/SnO2/Au-3 and, (f) rGO/SnO2/Au-4. (g) EDX pattern of the rGO/SnO2/Au-2 sample. | ||
Fig. 3 shows the Raman spectroscopy spectra confirming the reduction of GO. The two strong peaks of the D-band (∼1351 cm−1) and G-band (∼1595 cm−1) were observed, which correspond to the diamondoid and graphitic graphene structures, respectively. It is well known that the intensity ratio of the D and G band (ID/IG) is strongly related to the quantity of functional groups of rGO, and compared with the ID/IG value of GO (1.088), the increased value of rGO (1.453) suggests restoration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds after hydrothermal reduction.35
C bonds after hydrothermal reduction.35
Fig. 4 displays the typical TEM images of the rGO/SnO2/Au hybrid nanocomposite. The nanosheets have a size of several micrometers, with some surface wrinkles in some places. Compared with Fig. 4a, it can be observed in Fig. 4b that a large amount of SnO2 nanoparticle dots are attached on the surfaces of the nanosheets, with a size of about several nanometers, which is also reflected by the wide diffraction peaks of the XRD pattern. By close observation of Fig. 4c–f, some black dots with a darker contrast against the background can be observed, which are Au nanoparticles. It should be noted that Au nanoparticles can be distinguished from the nanoparticle dots. Interestingly, as the ratio of HAuCl4 increases, the size of Au nanoparticles also becomes larger and the sizes of the Au nanoparticles of rGO/SnO2/Au-1 to rGO/SnO2/Au-4 are about 12, 20, 42 and 90 nm, respectively.
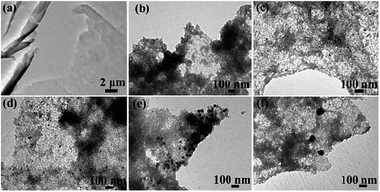 | ||
| Fig. 4 TEM images of the samples: (a) rGO, (b) rGO/SnO2, (c) rGO/SnO2/Au-1, (d) rGO/SnO2/Au-2, (e) rGO/SnO2/Au-3, (f) rGO/SnO2/Au-4. | ||
Fig. 5 reveals the typical TEM and the HRTEM images of the rGO/SnO2/Au-2 samples. Again the Au nanoparticles were clearly discovered to be located on the surface of the rGO/SnO2/Au-2 samples (Fig. 5a and b). Fig. 5c shows that interplanar distances of 0.23 nm and 0.333 nm correspond to the (111) planes of Au and (110) planes of SnO2.
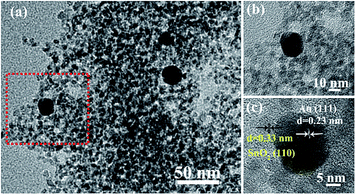 | ||
| Fig. 5 (a) TEM images of the rGO/SnO2/Au-2 samples and HRTEM images of (b) Au nanoparticles and (c) SnO2 nanoparticles. | ||
To further investigate the characteristics of the products, XPS technique was used to analyze the chemical state of GO and rGO/SnO2/Au nanocomposites. Fig. 6a and Fig. 6b reveal the C1s spectra of GO and rGO/SnO2/Au samples, exhibiting three peaks at 284.6, 286.6 and 288.4 eV, attributed to the C–C, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands in graphene-based materials.36 Note that the peak intensity of C–O and C
O bands in graphene-based materials.36 Note that the peak intensity of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is strong in GO. Compared with that, after hydrothermal treatment, the peak intensity of C–O and C
O is strong in GO. Compared with that, after hydrothermal treatment, the peak intensity of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O decreases remarkably, suggesting most oxygen-containing functional groups are successfully removed after hydrothermal treatment and Sn(IV). Fig. 6c shows the Sn 3d spectrum of rGO/SnO2/Au, showing two strong bands at 486.9 and 495.3 eV, which indicates the formation of SnO2.37 Fig. 6d confirms the presence of Au in the hybrids, with significant signals at 83.3 and 86.9 eV corresponding to metallic Au. A minor peak at 85.3 eV owing to Au(I) is also detected. The intensity of the Au 4f signal is higher than that of Au(I), indicating that most of Au is of zero valence and exits in the metallic form. All these observations indicate the successful formation of Au and SnO2 and reduction of GO by hydrothermal treatment of GO, SnCl2 and HAuCl4.
O decreases remarkably, suggesting most oxygen-containing functional groups are successfully removed after hydrothermal treatment and Sn(IV). Fig. 6c shows the Sn 3d spectrum of rGO/SnO2/Au, showing two strong bands at 486.9 and 495.3 eV, which indicates the formation of SnO2.37 Fig. 6d confirms the presence of Au in the hybrids, with significant signals at 83.3 and 86.9 eV corresponding to metallic Au. A minor peak at 85.3 eV owing to Au(I) is also detected. The intensity of the Au 4f signal is higher than that of Au(I), indicating that most of Au is of zero valence and exits in the metallic form. All these observations indicate the successful formation of Au and SnO2 and reduction of GO by hydrothermal treatment of GO, SnCl2 and HAuCl4.
 | ||
| Fig. 6 (a) C1s spectrum of GO (b) C1s spectrum of rGO/SnO2/Au-2 samples, (c) Sn3d spectrum of rGO/SnO2/Au-2, (d) Au4f spectrum of rGO/SnO2/Au-2. | ||
3.2. NO2 sensing properties
Sensors were fabricated by dropping the rGO/SnO2/Au dispersion onto the electrode to demonstrate NO2 sensing application. The effect of the amounts of Au nanoparticles on the sensing performance toward NO2 is investigated. Fig. 7 demonstrates the response/recovery curves of all rGO/SnO2/Au-based sensors to 5 ppm NO2 at 50 °C. It can be seen that the four sensors show relatively fast response and recovery speed. Notably, with the proportion of Au ranging from 0.5% to 5%. The maximum response with the value of 1.6 was obtained on rGO/SnO2/Au-2, So, the rGO/SnO2/Au-2 was chosen for the remaining test. | ||
| Fig. 7 The response curves to 5 ppm NO2 of the sensors based on rGO/SnO2/Au-1, rGO/SnO2/Au-2, rGO/SnO2/Au-3, and rGO/SnO2/Au-4. | ||
Moreover, the response/recovery speed is dramatically increased for the Au nanoparticles functionalized sensor. Fig. 8 shows the response and recovery times of the rGO, rGO/SnO2 and rGO/SnO2/Au-2-based sensors to 5 ppm NO2, respectively. The Tr1 values of rGO/SnO2/Au-2 sensors upon (Tr2 values during recovery after) exposure to 5 ppm NO2 gases are 19 s (20 s) at a low operating temperature of 50 °C, which are the shortest values in the literature (Fig. 8a). The Tr1 (Tr2) values of the rGO/SnO2 and pristine rGO sensors are relatively short 427 s (908 s) and 798 s (8319 s), (Fig. 8b and c). All these observations indicate that loading Au nanoparticles is an effective method for enhancing the response and response/recovery time (Tr1/Tr2) for graphene-based gas sensors at a low operating temperature.
 | ||
| Fig. 8 The response curve to 5 ppm NO2 of the sensors at 50 °C based on (a) rGO, (b) rGO/SnO2 and (c) rGO/SnO2/Au-2. | ||
Fig. 9 shows the response values of the rGO/SnO2/Au-2-based sensor being orderly exposed to 5–50 ppm NO2 gases at a low operating temperature of 50 °C. It is clear that the response and recovery characteristics were almost reproducible as well as the quick response and recovery times, which is in good agreement with the above analysis of Fig. 8c. Moreover, it is also found that the response gradually raises as the concentration of NO2 increases. The response values of the sensor to 5, 10, 20 and 50 ppm NO2 gas were about 1.67, 1.91, 2.37, and 2.68, respectively.
 | ||
| Fig. 9 Dynamic NO2 sensing transients curve of the rGO/SnO2/Au-2-based sensor to 5–50 ppm NO2 gases at 50 °C. | ||
We then investigated the response of three sensors at different NO2 concentrations (Fig. 10). The responses of Au-loaded sensors were linear from 5 to 30 ppm, but increased more slowly from 30 to 100 ppm as the sensor began to saturate. Significantly, it should be noted that the responses to NO2 were enhanced 5.85 times by employing rGO/SnO2/Au-2, in contrast with rGO/SnO2 and rGO. None-the-less, the sensor showed a broad range of linear response, this in turn, directly verifies the beneficial effect of Au nanoparticles.
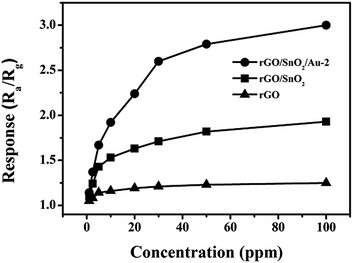 | ||
| Fig. 10 The responses of the rGO, rGO/SnO2, and rGO/SnO2/Au-2-based sensor to different concentrations of NO2 at 50 °C. | ||
The selectivity of three sensors was tested by exposing the sensors to potential interference gases (NO2, NO, Cl2, and H2), as shown in Fig. 11. Take 5 ppm target gases for example, the rGO/SnO2/Au-2-based sensor only gives a negligible response to interference gases, whereas the response of the rGO/SnO2/Au-2 sensor is significantly enhanced to NO2 gas compared with the rGO and rGO/SnO2 ones. The above results conclude that the rGO/SnO2/Au-2 has high selectivity for NO2 sensing.
 | ||
| Fig. 11 The responses of the rGO, rGO/SnO2 and rGO/SnO2/Au-2 based sensors to 5 ppm of different gases at 50 °C. | ||
Fig. 12 illustrates the reproducibility of the rGO/SnO2/Au-2 sensor, revealing that the sensor maintains its initial response amplitude without a clear decrease upon three successive sensing tests to 5 ppm of NO2, albeit the swift response and recovery process, indicating that the sensor has a good stability throughout the cycle test.
 | ||
| Fig. 12 The reproducibility of the rGO/SnO2/Au-2 sensor on successive exposure (3 cycles) to 5 ppm NO2 at 50 °C. | ||
3.3. Gas sensing mechanism of rGO/SnO2/Au
The possible sensing mechanism for the superior gas sensing performance is proposed as follows: firstly, the principle of gas sensing for the resistance-type sensors is based on the conductance variations of the sensing element, thus the introduction of Au and SnO2 to rGO significantly contributes to improving the conductivity, leading to a better sensing behavior. Secondly, benefiting from the existence of SnO2, the increasing number of dots facilitates the gas adsorption and diffusion on the active surface. In open air, the formation of oxygen adsorbates (O2−) on the surface of SnO2 and rGO results in an electron-depleted surface layer owing to electron transfer from the SnO2–rGOs to oxygen in a similar fashion as for the reported literature.38 Upon exposure to an oxidizing gas (NO2), gas molecules can easily adsorb on their active sites in the form of NO2 (Fig. 13). The Schottky barrier around the interface of rGO and SnO2 nanorods results in the specific capture and migration of electrons from SnO2 nanorods to graphene. As shown in Fig. 13, the role of rGO as an electron mediator further facilitates the electron transfer from SnO2 nanorods to NO2 molecules. Thirdly, the enhancement of NO2 gas sensing properties of rGO/SnO2 nanosheets by Au-functionalization can be explained based on the model proposed for the metal catalyst-enhanced gas sensing of nanomaterials.39 In the case of rGO/SnO2/Au nanorods, Au nanoparticles over the surface spill the NO2 gas over the rGO/SnO2 nanorods surface and enhance the chemisorption and dissociation of NO2 gas owing to the high catalytic or conductive nature of Au. Consequently, the number of electrons attracted to the gas increases. | ||
| Fig. 13 The scheme of the proposed gas sensing mechanism: the adsorption behaviour of NO2 molecules on the rGO/SnO2/Au-2 nanocomposite. | ||
4. Conclusions
rGO/SnO2/Au nanocomposites have been prepared by one-step hydrothermal synthesis method. The gas sensing results indicate that the introduction of the Au nanoparticles into the rGO/SnO2 composite materials significantly enhanced the NO2-sensing performance at a low operating temperature of 50 °C. The results showed that the rGO/SnO2/Au-2 sensors could detect NO2 gases as low as 1 ppm. The response and recovery times are 19 and 20 s to 5 ppm NO2. All results indicate that Au nanoparticles loading can significantly enhance the NO2 sensing properties of graphene-based sensing materials at a low operating temperature, which has excellent potential applications as gas sensors.Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China (Grant no. 51202085) and Program for Chang Jiang Scholars and Innovative Research Team in University (no. IRT1017).Notes and references
- R. Atkinson, Atmos. Environ., 2000, 34, 2063–2101 CrossRef CAS.
- D. Zhang, Z. Liu, C. Li, T. Tang, X. Liu, S. Han, B. Lei and C. Zhou, Nano Lett., 2004, 4, 1919–1924 CrossRef CAS.
- Y. Kanda and M. Taura, Anal. Chem., 1990, 62, 2084–2087 CrossRef CAS.
- D. V. Vinjamoori and C. S. Ling, Anal. Chem., 1981, 53, 1689–1691 CrossRef CAS.
- A. Aydm, Ö. Ercan and S. Tascioglu, Talanta, 2005, 66, 1181–1186 CrossRef CAS PubMed.
- A. K. Geim and A. H. MacDonald, Phys. Today, 2007, 60, 35–41 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva1 and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- M. J. Allen, V. C.Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132–145 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner and S. T. Nguyen, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
- S. Park and R. S.Ruoff, Nat. Nanotechnol., 2009, 4, 217–224 CrossRef CAS PubMed.
- S. Gilje, S. Han, M. Wang, K. L. Wang and R. B. Kaner, Nano Lett., 2007, 7, 3394–3398 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyenb and R. S. Ruoffa, Carbon, 2007, 45, 1558–1565 CrossRef CAS PubMed.
- D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- A. Lerf, H. Y. He, M. Forster and J. Klinowski, J. Phys. Chem. B, 1998, 102, 4477–4482 CrossRef CAS.
- F. Kim, L. J. Cote and J. Huang, Adv. Mater., 2010, 22, 1954–1958 CrossRef CAS PubMed.
- O. C. Compton and S. T. Nguyen, Small, 2010, 6, 711–723 CrossRef CAS PubMed.
- P. V. Kamat, J. Phys. Chem. Lett., 2010, 1, 520–527 CrossRef CAS.
- Y. Matsuo, K. Hatase and Y. Sugie, Chem. Mater., 1998, 10, 2266–2269 CrossRef CAS.
- Y. Matsuo, K. Tahara and Y. Sugie, Carbon, 1997, 35, 113–120 CrossRef CAS.
- Y. P. Dan, Y. Lu, N. J. Kybert, Z. T. Luo and A. T. C. Johnson, Nano Lett., 2009, 9, 1472–1475 CrossRef CAS PubMed.
- J. T. Robinson, F. K. Perkins, E. S. Snow, Z. Q. Wei and P. E. Sheehan, Nano Lett., 2008, 8, 3137–3140 CrossRef CAS PubMed.
- I. Jung, D. Dikin, S. Park, W. Cai, S. L. Mielke and R. S. Ruoff, J. Phys. Chem. C, 2008, 112, 20264–20268 CAS.
- G. H. Lu, L. E. Ocola and J. H. Chen, Appl. Phys. Lett., 2009, 94, 083111–083113 CrossRef PubMed.
- G. H. Lu, L. E. Ocola and J. H. Chen, Nanotechnology, 2009, 20, 445502 CrossRef PubMed.
- M. Shafiei, R. Arsat, J. Yu, P. G. Spizzirri, S. Dubin, R. B. Kaner, J. Plessis, K. Kalantar-zadeh and W. Wlodarski, J. Phys. Chem. C, 2010, 114, 13796–13801 CAS.
- N. Chen, X. G. Li, X. Y. Wanga, J. Yua, J. Wang, Z. N. Tang and S. A. Akbar, Sens. Actuators, B, 2011, 188, 902–908 CrossRef PubMed.
- W. S. Hummers and R. Offeman, J. Am. Chem. Soc., 1958, 80, 1339–1339 CrossRef CAS.
- J. Zhang, X. H. Liu, L. W. Wang, T. L. Yang, X. Z. Guo, S. H. Wu, S. M. Zhang and S. R. Wang, Carbon, 2011, 49, 3538–3543 CrossRef CAS PubMed.
- L. L. Wang, H. M. Dou, Z. Lou and T. Zhang, Nanoscale, 2013, 5, 2686–2691 RSC.
- L. L. Wang, T. Fei, Z. Lou and T. Zhang, ACS Appl. Mater. Interfaces, 2011, 3, 4689–4694 CAS.
- S. Dubin, S. Gilje, K. Wang, V. C. Tung, K. Cha, A. S. Hall, J. Farrar, R. Varshneya, Y. Yang and R. B. Kaner, ACS Nano, 2010, 4, 3845–3852 CrossRef CAS PubMed.
- S. M. Paek, E. Yoo and I. Honma, Nano Lett., 2009, 9, 72–75 CrossRef CAS PubMed.
- L. L. Wang, Z. Lou, T. Zhang, H. Fan and X. Xu, Sens. Actuators, B, 2011, 155, 285–289 CrossRef CAS PubMed.
- C. Y. Su, Y. Xu, W. Zhang, J. Zhao, X. Tang, C. H. Tsai and L. J. Li, Chem. Mater., 2009, 21, 5674–5680 CrossRef CAS.
- S. Pei, J. Zhao, J. Du, W. Ren and H. M. Cheng, Carbon, 2010, 48, 4466–4474 CrossRef CAS PubMed.
- Q. Zhao, Z. Zhang, T. Dong and Y. Xie, J. Phys. Chem. B, 2006, 110, 15152–15156 CrossRef CAS PubMed.
- G. H. Lu, L. E. Ocola and J. H. Chen, Adv. Mater., 2009, 21, 2487–2491 CrossRef CAS.
- A. Kolmakov, D. Klenov, Y. Lilach, S. Stemmer and M. Moskovits, Nano Lett., 2005, 5, 667–673 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |


