1,1′-Sulfinyldipyridinium bis (hydrogen sulfate) ionic liquid: synthesis and application in the temperature-influenced synthesis of novel pyranopyrimidinediones and pyranopyrimidinetriones†
Jayavant D. Patil,
Suyog N. Korade and
Dattaprasad M. Pore*
Department of Chemistry, Shivaji University, Kolhapur 416 004, India. E-mail: p_dattaprasad@rediffmail.com; Fax: +91 231 2692333; Tel: +91 231 2690571
First published on 25th September 2014
Abstract
A novel and robust dication homo-anionic ionic liquid with the properties of a Brønsted acid is shown to efficiently catalyse the reaction of salicylaldehyde and 6-amino uracil in ethanol to produce novel pyranopyrimidinediones and pyranopyrimidinetriones under ambient and reflux conditions, respectively. The ionic liquid can be synthesized from inexpensive, commercially available precursors. A mechanism is suggested for these transformations. The advantages of this method include novelty in terms of the ionic liquid, together with a high efficiency, easy work-up procedure and purification, convenient operation, mild and environmentally benign reaction conditions.
Introduction
Designing and exploring novel methods to synthesize pharmacological agents within the framework of green chemistry are common goals for organic chemists trying to reduce both costs and potential environmental problems.1 In this context, ionic liquids (ILs) have been proposed as both green solvents and as catalysts due to their unique chemical and physical properties, which include non-volatility, non-flammability, thermal stability and controlled miscibility.2 ILs are now recognized in organic reactions as providing potential improvements in the control of product distribution, enhanced reactivity, ease of product recovery, catalyst immobilization and recycling.3 ILs with sulfate-functionalized Brønsted acid sites have attracted attention because they are non-volatile, non-corrosive, stable in air and can be easily recovered and reused.4 ILs have been explored in many organic transformations as they offer advantages of both liquid and solid acids and they have thus emerged as useful alternatives to traditional mineral liquid acids, such as sulfuric acid and hydrochloric acid, in chemical reactions.5 Thus they have attracted the attention of researchers looking for new ILs for the synthesis of novel bioactive heterocyclic molecules.Pyrimidines are six-membered heterocyclic aromatic compounds with two nitrogen atoms at positions 1 and 3. Pyrimidine-embedded heterocyclic molecules are of great interest because they constitute an important class of natural and non-natural products, many of which have biological activity and clinical applications.6 Thymine, cytosine and uracil have a pyrimidine skeleton and are the building blocks of the nucleic acids DNA and RNA; they have widespread therapeutic applications. Some pyrimidines show significant in vitro activity against unrelated DNA and RNA, such as against the polio and herpes viruses; they also have diuretic, antitumour, anti-HIV and cardiovascular activities.7 In addition, various analogues of pyrimidines have been found to possess antibacterial,8 antifungal,9 antileishmanial,10 anti-inflammatory,11 analgesic,12 antihypertensive,13 antipyretic,14 antiviral,15 antidiabetic,16 antiallergic,17 anticonvulsant,18 antioxidant,19 antihistaminic,20 herbicidal21 and anticancer activities.22 Many pyrimidines have been reported to potentially possess properties to depress the central nervous system23 and they also act as calcium channel blockers.24 These varied activities have led to the discovery of a plethora of drugs with a pyrimidine backbone (Fig. 1). Previous investigations have shown that pyrimidine-immobilized heterocyclic molecules such as zidovudine (I) and lamivudine (II) can be used in the treatment of HIV25; brodiprim (III) and flucytosine (IV) are antibacterial and antifungal agents.26 Methylphenobarbital (V) is used as an antiepileptic drug27 and the 6-aryl uracil derivatives (VI) show antitumour activity.28 Brodiprim is an effective antibacterial treatment.29
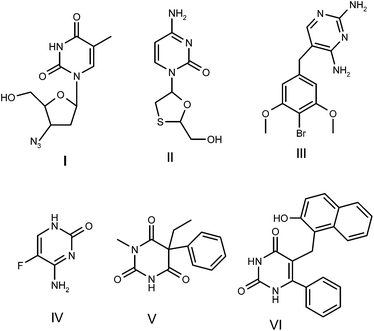 | ||
| Fig. 1 Drugs with pyrimidine backbones. I = zidovudine; II = lamivudine; III = brodiprim; IV = flucytosine; V = methylphenobarbital; and VI = 6-aryl uracil derivative. | ||
Results and discussion
As part of our continuing work in devising eco-friendly methodologies for the synthesis of the pyran nucleus,30 we report here the dramatic influence of a new task-specific IL for the synthesis of pyranopyrimidinediones and pyranopyrimidinetriones in an ethanol medium at room temperature and under reflux conditions, respectively (Scheme 1). | ||
| Scheme 1 Synthesis of the novel pyranopyrimidinedione and pyranopyrimidinetrione (products 4 and 5). | ||
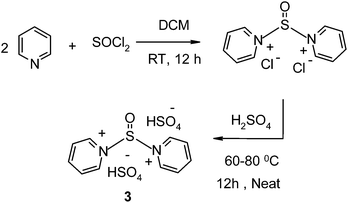
We first optimized the reaction conditions in terms of the catalyst for the model reaction of salicylaldehyde (1 mmol) and 6-amino uracil (2 mmol). The reaction was first carried out in the absence of a catalyst (entries 1 and 2, Table 1), but the reaction did not proceed, even under reflux conditions. Various Lewis and Brønsted acid catalysts were then screened for the formation of product 4 (Scheme 1). Lewis acids such as AlCl3, ZnCl2, FeCl3, and (CH2)4SO3Mim and Na p-TSA (entries 3–5, 13 and 15, Table 1) did not catalyse this transformation, even after long reaction times. Low yield of the desired product was obtained in the presence of p-TSA (entry 14, Table 1) and ILs such as [PySOCl]Cl, [PySOPy]Cl2, [BMim]HSO4, [PySOCl]HSO4 and [PySOCl]TSO (entries 6, 7 and 10–12, Table 1). These observations suggest that the yield of the reaction could be improved in the presence of a strong Brønsted acidic catalyst. Therefore ILs such as [(CH2)4SO3HMim]HSO4 and [CMim]HSO4 were used and yielded 65–75% of the desired product (entries 8 and 9, Table 1). The yield of the product was boosted to 84% in the presence of a catalytic amount of thionyl chloride (entry 16, Table 1). This inspired us to synthesize a novel IL with strong Brønsted acid sites together with thionyl (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) functionality to enhance the rate of the reaction. ILs based on pyridinium salts have attracted particular attention as they are easy to prepare and handle, have good solubility for many substrates and act as molecular catalysts. The synthesis of [(Py)2SO][HSO4]2 (hydrophilic IL 3) was achieved in two steps. [(Py)2SO]Cl2 was first prepared by the reaction of pyridine with thionyl chloride in dichloromethane, followed by treatment with sulfuric acid to give the desired IL in a quantitative yield (Scheme 2).
O) functionality to enhance the rate of the reaction. ILs based on pyridinium salts have attracted particular attention as they are easy to prepare and handle, have good solubility for many substrates and act as molecular catalysts. The synthesis of [(Py)2SO][HSO4]2 (hydrophilic IL 3) was achieved in two steps. [(Py)2SO]Cl2 was first prepared by the reaction of pyridine with thionyl chloride in dichloromethane, followed by treatment with sulfuric acid to give the desired IL in a quantitative yield (Scheme 2).
| Entry | Catalyst | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Salicylaldehyde (1 mmol), 6-amino uracil (2 mmol) and 20 mol% catalyst in ethanol at room temperature.b Isolated yield.c Reflux conditions. | |||
| 1 | No catalyst | 3 | — |
| 2 | No catalyst | 3 | —c |
| 3 | AlCl3 | 3 | — |
| 4 | ZnCl2 | 3 | — |
| 5 | FeCl3 | 3 | — |
| 6 | [PySOCl]Cl | 2 | 20 |
| 7 | [PySOPy][Cl]2 | 2 | 30 |
| 8 | [(CH2)4SO3HMim][HSO4] | 1 | 76 |
| 9 | [CMim][HSO4] | 1 | 66 |
| 10 | [BMim][HSO4] | 3 | 50 |
| 11 | [PySOCl][HSO4] | 2 | 53 |
| 12 | [PySOCl]TSO | 3 | 25 |
| 13 | (CH2)4SO3Mim | 3 | — |
| 14 | p-TSA | 2 | 45 |
| 15 | Na-PTSA | 3 | — |
| 16 | SOCl2 | 1 | 84 |
| 17 | [PySOPy][HSO4]2 | 1 | 96 |
The dicationic homo-anionic IL had exceptional catalytic activity and provided the corresponding product at an excellent yield as a result of its combined Lewis acid and Brønsted acid functionalities (entry 17, Table 1). The effect of the amount of catalyst was evaluated by using 5, 10, 15, 20, 25 mol% of the IL in the model reaction, which gave product 4 at 40, 70, 84, 96 and 96% yields, respectively. It was found that 20 mol% of the catalyst provided maximum yield. More than 20 mol% of the catalyst did not further improve the yield of the product or the reaction time. The performance of the reaction was also assessed using different solvents, such as chloroform, acetonitrile, DCM, acetone, DMF and THF. Non-polar solvents gave lower yields, even after increased reaction times (entries 1–4, Table 2). Ethanol was the best choice for this reaction when using 20 mol% of the catalyst (entry 9, Table 2).
As the reaction proceeds, a nearly homogeneous mixture is formed (Fig. 2A) and the product is precipitated from the ethanol (Fig. 2B). The product was isolated by simple filtration and confirmed by spectral analysis. The 1H-NMR spectrum (entry a, Table 3) shows remarkable singlets at δ = 4.81 and 5.77 ppm representing the benzylic methine proton and two amine protons, respectively. In the IR spectrum, the absorption bands at 3445, 3388 cm−1 represent the presence of a primary amine and 1695, 1634 cm−1 correspond to the carbonyls of amides, confirming the structure of product 4.
 | ||
| Fig. 2 (A) Reaction mixture at the start of the reaction and (B) reaction mixture after completion of the reaction. | ||
| Entry | Productb (4) | Time (h) | Yieldc (%) |
|---|---|---|---|
| a Reaction conditions: salicylaldehyde (1 mmol); 6-amino uracil (2 mmol); IL 20 mol%; temp = room temp; solvent = 5 mL 95% ethanol.b All products showed satisfactory spectroscopic data (IR, 1H-NMR, 13C-NMR and MS).c Yields refer to pure, isolated products. | |||
| a | R = H | 1 | 96 |
| b | R = 5-CH3O | 1 | 96 |
| c | R = 3-CH3CH2O | 1 | 98 |
| d | R = 3-OH | 1 | 94 |
| e | R = 4-OH | 1 | 92 |
| f | R = 5-OH | 1 | 94 |
| g | R = 3,4-OH | 1 | 92 |
| h | R = 5-Cl | 1.2 | 95 |
| i | R = 5-Br | 1 | 96 |
| j | R = 5-NO2 | 1.5 | 93 |
| k | R = naphthyl | 1.5 | 90 |
This serendipitous result led us to further explore the scope and general utility of this novel transformation. A series of salicylaldehydes was treated with 6-amino uracil under the optimized reaction conditions. Both electron-donating (entries b–g, Table 3) and electron-deficient (entries h–k, Table 3) salicylaldehydes gave the corresponding pyranopyrimidinediones in excellent yield (>82–96%), indicating an insignificant stereo-electronic effect (entries a–k, Table 3).
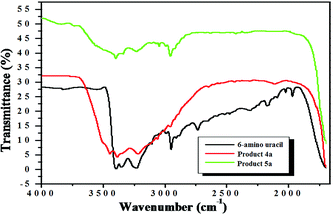
Having succeeded in the synthesis of pyranopyrimidinediones, we investigated the influence of temperature and performed the reaction of salicylaldehyde with 6-amino uracil in 95% ethanol at reflux temperatures in presence of 20 mol% IL 3 (Scheme 3). However, instead of the expected product 7, we observed the unexpected formation of the novel pyranopyrimidinetrione 5 at 87% yield (Scheme 3). Fig. 3 shows the IR spectrum of 6-amino uracil and the products 4 (entry a, Table 3) and 5 (entry a, Table 4). The bands due to the –NH2 in 6-amino uracil and the product 4 (entry a, Table 3) at 3445 and 3388 cm−1 disappear in product 5 (entry a, Table 4) and confirmed its formation. The pyranopyrimidinediones 4a–4k and the pyranopyrimidinetrione 5a–5d are unequivocally confirmed by FT-IR, 1H-NMR, 13C-NMR and MS analysis. The structure of 5 (entry a, Table 4) was further confirmed by X-ray crystallography, which showed the geometry of the crystal to be monoclinic with the two rings twisted out-of-plane to minimize the steric interactions (Fig. 4) 31
| Entry | Productb (5) | Time (h) | Yieldc (%) |
|---|---|---|---|
| a Reaction conditions: salicylaldehyde (1 mmol); 6-amino uracil (2 mmol); IL 20 mol%; temp = 80 °C; solvent = 5 mL of 95% ethanol.b All products showed satisfactory spectroscopic data (IR, 1H-NMR, 13C-NMR and MS).c Yields refer to pure, isolated products.d For electron-withdrawing substituents, the formation of product 4 was observed instead of product 5. | |||
| a | R = H | 5 | 94 |
| b | R = 5-CH3O | 6 | 92 |
| c | R = 3-CH3CH2O | 6 | 94 |
| d | R = 5-OH | 6 | 92 |
| ed | R = 5-NO2 | 10 | 80 |
| fd | R = 3,5-Cl | 10 | 84 |
 | ||
| Fig. 4 ORTEP diagram of product 5a (Table 4). | ||
To explore the scope of the reaction, other salicylaldehydes were tested and the results are summarized in Table 4. We observed that the electron-rich salicylaldehydes underwent a smooth reaction with 6-amino uracil to give the desired pyranopyrimidinetriones at excellent yields. In the case of the electron-deficient salicylaldehydes, product 4 was obtained instead of product 5, even after prolonged heating. We were unable to explain this anomaly.
A speculative mechanism for formation of products 4 and 5 is shown in Scheme 4. The synergistic combination of the acidic group and the thionyl group are the key factors in increasing the electrophilicity of the carbonyl carbon of 1, favoring the nucleophilic attack of 2. This is followed by ring closure, resulting in the formation of intermediate 6. The protonation of 6 by the IL and the subsequent Michael attack of the second molecule of 2 results in the formation of the desired product 4. The protonation of the amino group by IL 3 under thermal conditions is followed by the nucleophilic attack of water, yielding the corresponding product 5 by keto–enol tautomerization.
Recycling of the catalyst is one of the most significant criteria of green chemistry, hence the recovery and reuse of the IL catalyst was examined. The separation of the product was very easy and could be achieved by filtration through an ordinary filter paper. The IL was conveniently recovered and reused after heat treatment under vacuum at 70 °C for 2 h. The reusability of the recovered catalyst was studied in a fresh reaction and it was found that the IL could be reused at least five times without a significant decrease in the reaction yield (96, 93, 90, 89 and 88% yield, respectively) (Fig. 5).
Conclusion
We introduced a temperature-influenced IL-catalysed reaction of salicylaldehyde and 6-amino uracil that has led to a new class of pyranopyrimidinediones and pyranopyrimidinetriones. A detailed reaction mechanism is suggested. In general, all the reactions are very clean and reasonably fast. This method offers marked improvements with regard to operational simplicity, reaction time, mild reaction conditions, general applicability, high isolated yields of products, greener procedure, avoiding hazardous organic solvents and effective reusability of IL up to five runs without any loss of activity is a bonus advantage of present protocol.Experimental section
The various substituted salicylaldehydes (Sigma-Aldrich) and 6-amino uracil (Sigma-Aldrich) were used as received. The IR spectra were recorded on a Perkin-Elmer FT-IR-783 spectrophotometer. The NMR spectra were recorded on a Bruker AC-300 (300 MHz for 1H-NMR and 75 MHz for 13C-NMR) spectrometer in DMSO-d6 and CDCl3 using TMS as an internal standard; the δ values are expressed in ppm. Single-crystal X-ray crystallography was performed on a Bruker Kappa APEX II (SAIF, IIT, Madras).General procedures
Acknowledgements
Author DMP thanks the DST Fast Track Scheme New Delhi for financial assistance [no. SB/FT/CS-154/2012].References
- (a) C. W. Lindsley, Z. Zhao, W. H. Leister, J. O'Brien, W. Lemaire, D. L. Williams, T.-B. Chen, R. S. L. Chang, M. Burno, M. A. Jacobson, C. Sur, G. G. Kinney, D. J. Pettibone, P. R. Tiller, S. Smith, N. N. Tsou, M. E. Duggan, P. J. Conn and G. D. Hartman, ChemMedChem, 2006, 1, 807 CrossRef CAS PubMed; (b) E. Addicks, R. Mazitschek and A. Giannis, ChemBioChem, 2002, 3, 1078 CrossRef CAS; (c) W. Li, Y. Chang, P. Zhan, N. Zhang, X. Liu, C. Pannecouque and E. D. Clercq, ChemMedChem, 2010, 5, 1893 CrossRef CAS PubMed; (d) N. Richter, R. C. Simon, W. Kroutil, J. M. Wardd and H. C. Hailes, Chem. Commun., 2014, 50, 6098 RSC; (e) X. Wang, Y. Pan, X. Huang, Z. Mao and H. Wang, Org. Biomol. Chem., 2014, 12, 2028 RSC; (f) K. Arya, P. Tomar and J. Singh, RSC Adv., 2014, 4, 3060 RSC; (g) A. Kumar, G. Gupta, S. Srivastava, A. K. Bishnoi, R. Saxena, R. Kant, R. S. Khanna, P. R. Maulik and A. Dwivedi, RSC Adv., 2013, 3, 4731 RSC; (h) S. Singh, L. K. Sharma, A. Saraswat, I. R. Siddiqui, H. K. Kehri K Rana and P. Singh, RSC Adv., 2013, 3, 4237 RSC; (i) V. L. Pietra, G. L. Regina, A. Coluccia, V. Famiglini, S. Pelliccia, B. Plotkin, H. Eldar-Finkelman, A. Brancale, C. Ballatore, A. Crowe, K. R. Brunden, L. Marinelli, E. Novellino and R. Silvestri, J. Med. Chem., 2013, 56, 10066 CrossRef PubMed; (j) M. A. Toledo, C. Pedregal, C. Lafuente, N. Diaz, M. A. Martinez-Grau, A. Jiménez, A. Benito, A. Torrado, C. Mateos, E. M. Joshi, S. D. Kahl, K. S. Rash, D. R. Mudra, V. N. Barth, D. B. Shaw, D. McKinzie, J. M. Witkin and M. A. Statnick, J. Med. Chem., 2014, 57, 3418 CrossRef CAS PubMed; (k) X. Liu, Y. Wang, Y. Xing, J. Yu, H. Ji, M. Kai, Z. Wang, D. Wang, Y. Zhang, D. Zhao and R. Wang, J. Med. Chem., 2013, 56, 3102 CrossRef CAS PubMed; (l) M. Pettersson, D. S. Johnson, C. Subramanyam, K. R. Bales, C. W. am Ende, B. A. Fish, M. E. Green, G. W. Kauffman, P. B. Mullins, T. Navaratnam, S. M. Sakya, C. M. Stiff, T. P. Tran, L. Xie, L. Zhang, L. R. Pustilnik, B. C. Vetelino, K. M. Wood, N. Pozdnyakov, P. R. Verhoest and C. J. O'Donnel, J. Med. Chem., 2014, 57, 1046 CrossRef CAS PubMed.
- (a) T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed; (b) K. R. Seddon, Kinet. Catal., 1996, 37, 693 CAS; (c) S.-G. Lee, Chem. Commun., 2006, 1049 RSC; (d) N. Isambert, M. M. S. Duque, J.-C. Plaquevent, Y. G_nisson, J. Rodriguez and T. Constantieux, Chem. Soc. Rev., 2011, 40, 1347 RSC; (e) C. Lagrost, D. Carrie, M. Vaultier and P. Hapiot, J. Phys. Chem. A, 2003, 107, 745 CrossRef CAS; (f) H. Zhao, S. Xia and P. Ma, J. Chem. Technol. Biotechnol., 2005, 80, 1089 CrossRef CAS.
- (a) S. Safaei, I. Mohammadpoor-Baltork, A. R. Khosropour, M. Moghadam, S. Tangestaninejad and V. Mirkhani, Adv. Synth. Catal., 2012, 354, 3095 CrossRef CAS; (b) W. L. Wong, K. P. Ho, L. Y. S. Lee, K. M. Lam, Z. Y. Zhou, T. H. Chan and K. Y. Wong, ACS Catal., 2011, 1, 116 CrossRef CAS; (c) C. Yue, D. Fang, L. Liu and T. F. Yi, J. Mol. Liq., 2011, 163, 99 CrossRef CAS PubMed; (d) H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1 CrossRef CAS PubMed; (e) D. Li, F. Shi, J. Peng, S. Guo and Y. Deng, J. Org. Chem., 2004, 69, 3582 CrossRef CAS PubMed.
- (a) N. S. Gupta, G. L. Kad and J. Singh, Catal. Commun., 2007, 8, 1323 CrossRef CAS PubMed; (b) B. A. Da Silveira Neto, G. Ebeling, R. S. Goncalves, F. C. Gozzo, M. N. Eberlin and J. Dupont, Synthesis, 2004, 1155 CAS; (c) H. H. Wu, F. Yang, P. Cui, J. Tang and M. Y. He, Tetrahedron Lett., 2004, 45, 4963 CrossRef CAS PubMed; (d) A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis Jr, J. Am. Chem. Soc., 2002, 124, 5962 CrossRef CAS PubMed.
- (a) D. C. Forbes and K. J. Weaver, J. Mol. Catal. A: Chem., 2004, 214, 129 CrossRef CAS PubMed; (b) H. P. Zhu, F. Yang, J. Tang and M. Y. He, Green Chem., 2003, 5, 38 RSC; (c) K. Qiao and C. M. Yokoyama, Chem. Lett., 2004, 33, 472 CrossRef CAS; (d) R. G. Kalkhambkar, S. N. Waters and K. K. Laali, Tetrahedron Lett., 2011, 52, 867 CrossRef CAS PubMed; (e) X. Liang and J. Yang, Green Chem., 2010, 12, 201 RSC; (f) Y. Gu, J. Zhang, Z. Duan and Y. Deng, Adv. Synth. Catal., 2005, 347, 512 CrossRef CAS; (g) F. Dong, L. Jun, Z. Xinli, Y. Zhiwen and L. Zuliang, J. Mol. Catal. A: Chem., 2007, 274, 208 CrossRef CAS PubMed.
- (a) D. J. Brown, Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon Press, Oxford, UK, 1984, vol. 14 Search PubMed; (b) R. C. Elderfield, Heterocyclic Compounds, John Wiley & Sons, New York, NY, USA, 1957, vol. 6 Search PubMed.
- C. O. Kappe, Tetrahedron, 1993, 49, 6937 CrossRef CAS.
- (a) P. Sharma, N. Rane and V. K. Gurram, Bioorg. Med. Chem. Lett., 2004, 14, 4185 CrossRef CAS PubMed; (b) O. Prakash, V. Bhardwaj, R. Kumar, P. Tyagi and K. R. Aneja, Eur. J. Med. Chem., 2004, 39, 1073 CAS; (c) M. Botta, M. Artico and S. Massa, Eur. J. Med. Chem., 1992, 27, 251 CrossRef CAS; (d) N. Agarwal, P. Srivastava and S. K. Raghuwanshi, Bioorg. Med. Chem. Lett., 2002, 10, 869 CrossRef CAS.
- (a) N. Agarwal, S. K. Raghuwanshi, D. N. Upadhyay, P. K. Shukla and V. J. Ram, Bioorg. Med. Chem. Lett., 2000, 10, 703 CrossRef CAS; (b) H. S. Basavaraja, G. M. Sreenivasa and E. Jayachandran, Indian J. Heterocycl. Chem., 2005, 15, 69 Search PubMed.
- V. J. Ram, N. Haque and P. Y. Guru, Eur. J. Med. Chem., 1992, 27, 851 CrossRef CAS.
- (a) M. Amir, S. A. Javed and H. Kumar, Indian J. Pharm. Sci., 2007, 68, 337 CrossRef PubMed; (b) S. M. Sondhi, S. Jain, A. D. Dwivedi, R. Shukla and R. Raghubir, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2008, 47, 136 Search PubMed.
- S. Vega, J. Alonso, J. A. Diaz and F. Junquera, J. Heterocycl. Chem., 1990, 27, 269 CrossRef CAS.
- (a) D. R. Hannah and M. F. G. Stevens, J. Chem. Res., Synop., 2003, 7, 398 CrossRef; (b) K. Rana, B. Kaur and B. Kumar, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2004, 43, 1553 Search PubMed.
- P. A. S. Smith and R. O. Kan, J. Org. Chem., 1964, 29, 2261 CrossRef CAS.
- J. Balzarini and C. McGuigan, J. Antimicrob. Chemother., 2002, 50, 5 CrossRef CAS PubMed.
- H. W. Lee, Y. K. Bok and B. A. Joong, Eur. J. Med. Chem., 2005, 40, 862 CrossRef CAS PubMed.
- P. F. Juby, T. W. Hudyma, M. Brown, J. M. Essery and R. A. Partyka, J. Med. Chem., 1979, 22, 263 CrossRef CAS.
- A. K. Gupta, H. P. Sanjay Kayath, A. Singh, G. Sharma and K. C. Mishra, Indian J. Pharmacol., 1994, 26, 227 CAS.
- (a) A. A. Abu-Hashem, M. M. Youssef and H. A. R. Hussein, J. Chin. Chem. Soc., 2011, 58, 41 CrossRef CAS; (b) A. A. Abu-Hashem, M. F. El-Shehry and F. A. Badria, Acta Pharm., 2010, 60, 311 CrossRef CAS PubMed.
- S. A. Rahaman, Y. R. Pasad, P. Kumar and B. Kumar, Saudi Pharm. J., 2009, 17, 255 CrossRef PubMed.
- Y. Nezu, M. Miyazaki, K. Sugiyama and I. Kajiwara, Pestic. Sci., 1996, 47, 103 CrossRef CAS.
- (a) F. Xie, H. Zhao, L. Zhao, L. Lou and Y. Hu, Bioorg. Med. Chem. Lett., 2009, 19, 275 CrossRef CAS PubMed; (b) M. A. Kaldrikyan, L. A. Grigoryan, V. A. Geboyan, F. G. Arsenyan, G. M. Stepanyan and B. T. Garibdzhanyan, Pharm. Chem. J., 2000, 34, 521 CrossRef CAS.
- (a) A. L. S. Rodrigues, J. M. Rosa and V. M. Gadotti, Pharmacol., Biochem. Behav., 2005, 82, 156 CrossRef CAS PubMed; (b) J. Tani, Y. Yamada, T. Oine, T. Ochiai, R. Ishida and I. Inoue, J. Med. Chem., 1979, 22, 95 CrossRef CAS.
- B. Kumar, B. Kaur, J. Kaur, A. Parmar, R. D. Anand and H. Kumar, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2002, 41, 1526 Search PubMed.
- (a) J. Darbyshire, M. Foulkes, R. Peto, W. Duncan, A. Babiker, R. Collins, M. Hughes, T. Peto and A. Walker, The Cochrane Library, 2 John Wiley & Sons, Chichester, 2004 Search PubMed; (b) H. Mitsuya, K. J. Weinhold and P. A. Furman, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 7096–7100 CrossRef CAS.
- (a) J. Smith and D. Andes, Ther. Drug Monit., 2008, 30, 167 CrossRef CAS PubMed; (b) B. Chadwick, M. Addy and D. M. Walker, Br. Dent. J., 1991, 171, 83 CrossRef CAS.
- M. J. Eadie and W. D. Hooper, Other Barbiturates-Methylphenobarbital in Antiepileptic Drugs, ed. E. H. Levy, R. Mattson, B. S. Meldrum and E. Perucca, Lippincott-Williams and Wilkins, Philadelphia, 2002 Search PubMed.
- F. Medda, R. J. M. Russell, M. Higgins, A. R. McCarthy, J. Campbell, A. M. Z. Slawin, D. P. Lane, S. Lain and N. J. Westwood, J. Med. Chem., 2009, 52, 2673 CrossRef CAS PubMed.
- I. Kompis and A. Wick, Helv. Chim. Acta, 1977, 60, 3025 CrossRef CAS.
- (a) D. M. Pore, K. A. Undale, B. B. Dongare and U. V. Desai, Catal. Lett., 2009, 132, 104 CrossRef CAS; (b) D. M. Pore, T. S. Shaikh, K. A. Undale and D. S. Gaikwad, C. R. Chim., 2010, 13, 1429 CrossRef CAS PubMed; (c) K. A. Undale, Y. K. Park, K. M. Park, D. H. Dagade and D. M. Pore, Synlett, 2011, 6, 791 Search PubMed; (d) D. M. Pore, P. B. Patil, D. S. Gaikwad, P. G. Hegade, J. D. Patil and K. A. Undale, Tetrahedron Lett., 2013, 54, 5876 CrossRef CAS PubMed.
- ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1005214. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra10410b |
| This journal is © The Royal Society of Chemistry 2014 |