Chemically triggered release of 5-aminolevulinic acid from liposomes†
Abstract
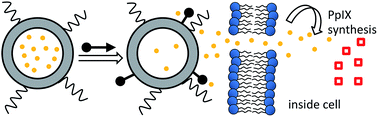
5-Aminolevulinic acid (5-ALA), a prodrug of protoporphyrin IX (PpIX), is used for photodynamic therapy of several medical conditions, and as an adjunct for fluorescence guided surgery. The clinical problem of patient photosensitivity after systemic administration could likely be ameliorated if the 5-ALA was delivered more selectivity to the treatment site. Liposomal formulations are inherently attractive as targeted delivery vehicles but it is hard to regulate the spatiotemporal release of aqueous contents from a liposome. Here, we demonstrate chemically triggered leakage of 5-ALA from stealth liposomes in the presence of cell culture. The chemical trigger is a zinc(II)-dipicolylamine (ZnBDPA) coordination complex that selectively targets liposome membranes containing a small amount of anionic phosphatidylserine. Systematic screening of several ZnBDPA complexes uncovered a compound with excellent performance in biological media. Cell culture studies showed triggered release of 5-ALA from stealth liposomes followed by uptake into neighboring mammalian cells and intracellular biosynthesis to form fluorescent PpIX.

 Please wait while we load your content...
Please wait while we load your content...