One-pot solvothermal synthesis of singly doped Eu3+ and codoped Er3+, Yb3+ heavy rare earth oxysulfide Y2O2S nano-aggregates and their luminescence study
Hong Liu†
a,
Ping Liu†a,
Xiangming Su†a,
Jie Liu*a,
Xiaoqing Lia,
Hongde Luoa,
Zhiling Yaoa,
Xibin Yu*a and
Ming Zhan*b
aThe Education Ministry Key Laboratory of Resource Chemistry and Shanghai Key Laboratory of Rare Earth Functional Materials, Department of Chemistry, Shanghai Normal University, Shanghai 200234, People's Republic of China. E-mail: liujie@shnu.edu.cn; xibinyu@shnu.edu.cn
bPhysics and Chemistry Test Lab, Shanghai Pudong New Area Center for Disease Control & Preverntion, Shanghai 200136, People's Republic of China. E-mail: mzhan@pdcdc.sh.cn
First published on 27th October 2014
Abstract
Highly crystallized micro/nano-sized Y2O2S and RE (Eu3+, Yb3+/Er3+) doped samples were prepared through a one-pot mild (200–250 °C) solvothermal method. By simply adjusting the amount of initial sulfur powder, the morphology and assembled nano-structures could be well controlled. With varying concentration of sulfur powder from 0.08 M to 0.8 M, the morphology and size of the target products varied from 20 nm nanosheets, 2 μm sheet-assembled plates/flowers to 1.5 μm nano-assembled spheres. The luminescence properties of Eu3+ doped Y2O2S with different morphologies were studied. The excitation peak above 300 nm ascribed to S2−–Eu3+ charge transfer showed a blue shift of 9 nm compared with the bulk sample. When co-doped with Yb3+/Er3+, the Y2O2S:Yb3+, Er3+ nano-aggregates exhibited a tunable emission under 980 nm laser excitation, shifting from greenish-yellow to orange-yellow, with increasing Yb3+/Er3+ ratio.
1 Introduction
Due to the high absorption of UV light and efficient energy transfer, high thermal and chemical stability, rare earth oxysulfides have been extensively used as optical functional materials. Among these materials, rare earth ion-activated Y2O2S has been used in many applications. Eu3+ ion doped Y2O2S is one of the most efficient phosphor that used in the field of color television tubes,1 long-lasting phosphor,2 filed emission display3 and photocatalysis.4 Y2O2S is also an efficient upconversion host for Yb3+/Er3+, Yb3+/Tm3+, Yb3+/Ho3+.Recently, there is much interest in nano crystalline rare earth oxysulfides synthesized through soft chemical method because of the unique luminescence and magnetics properties resulting from nano size and surface effect.5–8 As we know, it is difficult to obtain lanthanide oxysulfides directly through some soft synthetic method such as hydrothermal reaction and precipitation, especially for the heavy RE element such as yttrium, because the hard Lewis acid Ln3+ doesn't preferentially bind to the soft Lewis base S2−.9 In the previous references, some light and middle RE oxysulfides can be synthesized through the thermolysis method in high-boiling-point organic solvent.10 However, according to the density functional theory calculation results, from light rare earth to heavy rare earth, the thermo-dynamical stability are being lower, higher chemical potential of sulphur is needed to synthesize the oxysulfide. So, it is very difficult to prepare heavy RE oxysulfides in an one-pot soft chemistry rote. Post annealing treatment is necessary to form the Y2O2S phase and increase the crystallinity. But, such rigorous reaction conditions tend to result in aggregates of nanoparticles and difficulty in controlling the particle size and dispersity. Therefore, the synthesis of heavy RE oxysulfides with high crystallinity still remains a challenge for researchers to date. Yan et al. reported a synthesis of Y2O2S:Eu nanoplates through thermolysis method, but the preparation demands use of H2S gas and acetylacetonates in an oxygen-free environment, the crystallinity of product is relatively low.11
In this paper, the heavy RE oxysulfide Y2O2S has been prepared through a solvothermal reaction method. Ethanediamine and sulphur powder are used as the main solvent and sulphur source to get a higher chemical potential. If PVP are used as capping agents, the monodispersed Y2O2S nano-sheets aggregates are obtained. Furthermore, the amount of sulphur powders also play an important role in controlling the multi-morphologies. Y2O2S nano-particles evolve from ultra-thin nanosheets, flower-like structures to solid nano-spheres, with increasing the amount of sulfur. The rare earth doped samples show good luminescent properties without post-calcination treatment. As examples, the luminescent properties of singly doped Eu3+ and codoped Er3+, Yb3+ heavy rare earth oxysulfide Y2O2S are investigated.
2 Experimental
2.1 Materials
Yttrium acetate (Y(CH3COO)3·4H2O) (99.99%) was purchased from Shanghai Diyang Chemicals, the rare-earth nitrates, including Eu(NO3)3·6H2O (99.9%), Er(NO3)3·6H2O (99.9%), Yb(NO3)3·6H2O (99.9%) and other chemicals (analytical grade purity) were purchased from Aladdin and used without any further purification.2.2 Methods
In a typical procedure, 0.67 mmol Y(CH3COO)3·4H2O was added into 5 ml absolute ethanol and dissolved completely. Then, 2 g PVP (K29–32) was slowly added into the above solution. After vigorous stirring for 30 min, the above solution was drop wise added into 30 ml ethylenediamine solution containing 0.205 g sulfur powder (99.99%). The prepared solution was vigorously stirred for another 30 min. Then it was transferred to a 50 ml Teflon-lined stainless autoclave and heated at 250 °C for 24 h. For the luminescent samples, a certain amount of rare-earth nitrates including Eu(NO3)·6H2O, Er(NO3)3·6H2O and Yb(NO3)3·6H2O was added together with Y(CH3COO)3·4H2O. The ambient temperature was around 25 °C.2.3 Characterization
The X-ray powder diffraction (XRD) pattern was recorded using a Japan Regaku D/max çA X-ray diffractometer equipped with graphite monochromatized Cu Kα radiation (λ = 1.5418 Å) irradiated at a scanning rate of 4° min−1. The field-emission scanning electron microscopy (FESEM) images were obtained using a JEOL JSM-7500F microscope operated at an acceleration voltage of 15 kV. A JEOL JEM-200CX microscope operating at 160 kV in the bright-field mode was used for transmission electron microscopy (TEM). The selected area electron diffraction (SAED) pattern was performed on a JEOL JEM-2010 electron microscope operating at 200 kV. The photo-excitation (PLE) and photo-luminescence (PL) spectra were measured using a Varian spectrometer and performed at room temperature. Upconversion (UC) spectra were recorded on a Varian spectrometer equipped with an external power-controllable 980 nm semiconductor laser (Beijing Viasho Technology Company, Beijing, China) as the excitation source.3 Results and discussion
3.1 Phase Identification and morphology
Fig. 1 shows the XRD patterns of the products prepared at different temperatures for 16 h. The result shows that XRD pattern of sample prepared at 180 °C exhibits a different pattern compared with the JCPDS card 24-1424 of Y2O2S, we believe this product should be YOOH according to the JCPDS card 20-1413 of YOOH. When the temperature increases above 200 °C, the clear XRD peaks are well matched with the hexagonal Y2O2S phase. | ||
| Fig. 1 XRD patterns of the Y2O2S samples obtained after different solvothermal treatment temperature of 180, 200, 220, 250 °C for 16 h. | ||
To investigate the growing process, the XRD patterns of products collected after different solvothermal treatment times of 0.5, 2, 8, 16 and 24 h with other parameters being constant are shown in Fig. 2. The products present a broad band after 0.5 h of reaction, indicating it is amorphous and has a low degree of crystallinity. After 2 h or more time of treatment, the peaks are clear and well indexed as the JCPDS 24-1424 Y2O2S phase. The XRD results show that the hexagonal Y2O2S phase is prepared directly in solvothermal reaction system. As we know, it is difficult to obtain lanthanide oxysulfides, especially heavy rare earth oxysulfides, through some soft synthetic method such as solvothermal reaction and precipitation, because the hard Lewis acid Ln3+ doesn't preferentially bind to the soft Lewis base S2−.11
 | ||
| Fig. 2 XRD patterns of the Y2O2S samples obtained after different solvothermal treatment time of 0.5, 2, 8, 16, 24 h for 250 °C. | ||
In order to solve this problem, we have tried to increase the reaction activity of S2+ ions. Ethanediamine and sulphur powder are used as the main solvent and sulphur source to get a higher chemical potential. In the dissolution, a large amount of sulfur forms a complex with ethanediamine and the sulfur ring (S8) is changed into sulfur chains (–S–S–). As the temperature is increased, the sulfur polyanions, e.g. S62−, S42−, can be produced. These polyanions are not stable at high temperature and dissociate, from which required S2− ions are released. The newly produced S2− in high concentration overcome the low affinity of S2− with Y3+ to a great extent. As a result, Y2O2S could be synthesized directly in this solvothermal reaction system with a relatively low temperature and higher crystallization compared with other Y2O2S samples obtained through soft synthetic method reported before.
In addition, ethanol is used as secondary solvent in this method to improve the solubility of the Y(CH3COO)3·4H2O and the surfactant. The amount of ethanol should be controlled less than 5 ml in a 40 ml solvent in total. This ratio is quite lower than the synthesis of Gd2O2S reported by us before,12 this phenomenon can be explained that the synthesis of heavy RE element yttrium oxysulfide needs a higher S2− concentration to offer enough sulfur chemical potential.
The morphology of a typical Y2O2S nano-aggregates sample is shown in Fig. 3. As shown in Fig. 3(a), uniform plate-like aggregates on a large scale are synthesized. The sample shows good dispersibility and a uniform diameter of 2 μm. Detailed observation characterization is shown in Fig. 3(b–e) by TEM. In Fig. 3(b), it's implied that the plate-like aggregates consisted of nano sheets. From the amplification of the aggregates' edge in Fig. 3(c), it's found that the diameter of nano-sheet is 30–50 nm and the thickness is 6–8 nm. Another angle of the nanosheet is shown in Fig. 3(d), an enlarged HRTEM of the selected area in Fig. 3(d) is shown in Fig. 3(e), the lattice fringes image matches well with characteristics of the hexagonal Y2O2S crystal, the d spacing of 0.324 nm corresponds to the distance between (100) planes. The inset Fig. 3(f) shows the SAED taken along the [001] zone axis, and a regular hexagonal diffraction pattern indicated that the sample has a hexagonal crystal structure. It can be concluded that nano sheet grows along the [100] crystallographic direction and enclosed by ±(001) facets. This phenomenon is ascribed to the orientation function of the ethanediamine.
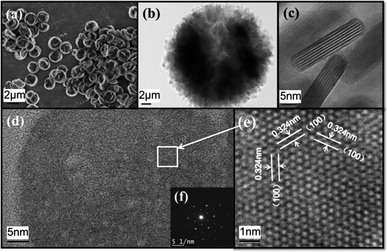 | ||
| Fig. 3 (a): FESEM of monodisperse Y2O2S nano-aggregates; (b–e): high and low magnification TEM images of Y2O2S nano-aggregates; inset (f) is the SAED pattern. | ||
The dispersion effect of using PVP as surfactant is shown in Fig. 4. When no surface active agent is used, the product is amorphous cross-linked network structure shown in Fig. 4(a1 and a2), but it's obviously consisting of curved nano sheet with a very thin thickness of below 10 nm. When 2 g PVP is used as surfactant, it's clearly implied that Y2O2S nano sheet shows an orientation of aggregation. The existence of PVP doesn't change the nanoscale sheet morphology of Y2O2S. The selective adsorption on the certain crystal plane of PVP is not as effective as ethanediamine. The viscosity of the solution system is increased by PVP, the cross-linked network structure is unable to be formed because of the barrier effect. The aggregation of nanoscale sheet Y2O2S is limited within a small range.13 Firstly the nanoscale sheet assembles into larger lamellar structure, then uniform micro-plate structure is assembled. After all, the homogeneity and dispersion of the Y2O2S nano-aggregates are enhanced efficiently by using PVP as surfactant.
 | ||
| Fig. 4 FESEM of the Y2O2S samples obtained from (a1 and a2) without PVP; (b1 and b2) with 2 g PVP with the same solvothermal treatment time at 250 °C for 24 h. | ||
Fig. 5 shows different morphology results by simply changing the concentration of sulfur powder when other parameters being constant. When the concentration of sulfur powder is as low as 0.08 M, Fig. 5(a1 and a2) shows that Y2O2S nano-sheets are obtained, and the thickness and diameter are ∼5 nm and ∼20 nm, respectively. With the sulfur concentration increased to 0.16 M, Y2O2S nano-sheets are assembled into a plate like structure with a diameter of ∼2 μm, shown in Fig. 5(c1 and c2). When the sulfur concentration is further increased to 0.4 M, much more Y2O2S nano-sheets are assembled into a flower like structure with a diameter of ∼2 μm. It is easy to find that the sheets structure is maintained, if the sulfur concentration is not too high. However, when the concentration of sulfur powder reaches 0.8 M, the morphology and structure of Y2O2S product change greatly. Sheets structure disappears and the morphology turns into nano particles-assembled solid spheres with a diameter of 1.5 μm.
 | ||
| Fig. 5 FESEM and TEM of the Y2O2S samples obtained from different initial sulfur concentration (a1 and a2): 0.08 M; (b1 and b2): 0.16 M; (c1 and c2): 0.4 M; (d1 and d2): 0.8 M at 250 °C for 24 h. | ||
According to the Y2O2S morphology under different reaction conditions, we conclude a morphology control rule in this reaction system as shown in Fig. 6. In the initial stage of reaction, small hexagonal particles of Y2O2S are obtained after nucleation and crystal growth under the oriented effect of the solvent and surfactant. If the concentration of sulfur powder is too high (0.8 M), nucleus number is relatively higher, the growth speed of small particles is slower than accumulation reunion's, therefore the particles will aggregate into dense sphere structure.
On the other hand, if the concentration of sulfur powder is very low (0.08 M), nucleus growth occupied the dominant position, hexagonal particles will assembled into nano sheet with a thickness of ∼5 nm, because this condition is difficult to control in the solvothermal system, the reunion phenomenon will appear to some extent. When the concentration of sulfur powder is in the middle value, nano sheet will be assembled into larger size nanosheet, the size growth will compete with assembling, when the concentration of sulfur powder is relatively lower, size growth will be in the first place and micrometer-grade plate assembled with larger nanosheets will be obtained. On the contrary, micrometer grade flower-like structure assembles with smaller nanosheets will be obtained.
3.2 Photoluminescence properties
Due to low phonon energies, low symmetry, outstanding chemical stability and nontoxicity, Y2O2S is an ideal luminescence host which is used in CRT imaging, cathode ray luminescence, upconversion phosphor and many other areas.In this paper, Eu3+ is doped in Y2O2S nano-aggregates with the best doping rate of 1%. Room temperature excitation spectra of Y2O2S:Eu3+ nano-aggregates and bulk Y2O2S:Eu3+ synthesized at 1050 °C for 4 h, monitored at 627 nm, are shown in Fig. 6. As we know, the excitation band from 250 to 300 nm can be ascribed to O2− to Eu3+ charge transfer and the broad excitation band above 300 nm is ascribed to S2− to Eu3+ charge transfer in this system.14 The excitation band width exhibits a broad trend in Y2O2S:Eu3+ nano-aggregates compared with the bulk sample, this may be ascribe to the increase of defects and surface effect of nano materials. At the same time, the excitation band above 300 nm shows a blue-shift of 9 nm in nano-aggregates, the similar phenomenon is observed in several ref. 9 Yan and co-workers synthesized Y2O2S:Eu3+ through thermolysis method, it's found that the peak of excitation band is 251 nm with several lower peaks above 300 nm because of the specific nano plate structure, S2− to Eu3+ charge transfer is not so effective. In this paper, Y2O2S:Eu3+ nano-aggregates are consist of nano sheet, since the nano sheet is terminated by Y3+ on both (001) facets, the S2− ion on the surface is not as many as that in bulk sample, as a result, the peak above 300 nm shifted to 325 nm in nano-aggregates. The main emission peaks are ascribed to the Eu3+ ion transition from 5DJ (J = 0, 1) to 7FJ (J = 1–4). The red/orange value is much higher in the nano-aggregates material and the emission peaks intensity (intensity = 20.7) decrease, compared to the bulk sample (intensity = 100). The lower crystal field symmetry at the surface is considered to be the main origin of the unusual phenomenon.10 It is already known that a blue shift in the Eu3+ ligand charge transfer band can lead to enhancement in the 5D0 → 7F2 transition.15 As a result, the ratio of peaks intensity responding to 5D0 → 7F2 transition and 5D0 → 7F1 or other transition is much higher in Y2O2S:Eu3+ nano-aggregates compared to bulk sample.
Excitation and emission spectra of different morphologies are also shown in Fig. 7. The sphere nano-aggregate Y2O2S:Eu3+ shows the strongest peaks intensity while the other three morphologies of Y2O2S:Eu3+ show the similar intensity. This phenomenon can be ascribed to the sphere nano-aggregates have the lowest surface defects and highest light absorption for the sphere structure. While all other three morphologies consisted of similar nano sheets which have a similar luminescence properties, but the flower-like nano-aggregates is closer to the sphere structure, the nano sheet exposes more defects, the former has a relatively strong intensity and the latter's is the lowest.
The upconversion luminescent properties of Yb3+/Er3+ doped Y2O2S is studied by adjusting the doping concentration. The sphere morphology of Y2O2S nano-aggregates is used as the host. The emission spectra when excited with 980 nm pumped laser is shown in Fig. 8. By adjusting the doping ratio of Er3+/Yb3+, the green/red luminescence ratio can be adjusted effectively. With the increase of Er3+ ion concentration, the green light component increased.
 | ||
| Fig. 8 Upconversion luminescence properties of Yb3+/Er3+ doped Y2O2S nano-aggregates with varying doping concentration under 980 nm laser excitation. | ||
The relationship between peak intensity and the laser pump power can be presented in formula of I ∝ Pn (n ≥ 2), therefore the photon number in one conversion process can be calculated. As logarithmic plot shown in Fig. 9, for 527 nm (2H11/2 → 4I15/2), 547 nm (4S3/2 → 4I15/2) and 667 nm (4F9/2 → 4I15/2), the slopes are 1.84, 1.82 and 1.89 respectively which prove that all the three conversion process absorb two low-energy photons and emit one high-energy photon.
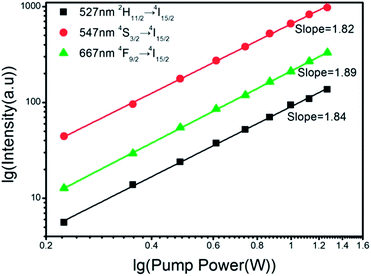 | ||
| Fig. 9 Power dependence of UC intensity (527, 547, 667 nm) of Y2O2S:10% Yb3+, 1% Er3+ nano-assembled spheres. | ||
Fig. 10 shows the energy level transition process of upconversion of Yb3+/Er3+ doped Y2O2S. There are two ways for Er3+ions to be inspired to the excited state 4I11/2 from the ground state 4I15/2: the first way is the ground state absorption (ESA) of Er3+ ion, and the second way is through the process of energy transfer (ETU) from Yb3+ ions: Er3+(4I15/2) + Yb3+(2F5/2) → Er3+(4I11/2) + Yb3+(2F7/2) which refers to that the energy absorbed by Yb3+ ion at the excited state are transmitted to the neighboring Er3+ ion because the energy is consistent. Obviously, the latter way dominated, because the absorption of Er3+ ions is poor at 980 nm excitation light source compared to the absorption intensity of Yb3+ ions. When Er3+ ion reach excited state 4I11/2, it can be excited to 4F7/2 level by another ETU process or ESA process. The Er3+ ion at 4F7/2 level will get to 2H11/2, 4S3/2 and 4F9/2 level through radiationless relaxation, and finally transit back to ground state level to emit the corresponding green light (527 nm, 547 nm) and red light (667 nm). This process is also consistent with the upconversion processes of two photon calculated before.
4 Conclusions
In summary, the heavy rare earth oxysulfide Y2O2S is directly synthesized by a facile and mild solvothermal reaction method for the first time. Ethanediamine, which dissolves sulphur powder and releases active S2 ions in high concentration, is used to overcome the low affinity of S2− ion with Y3+ ion. By simply adjusting the amount of initial sulfur powder, the morphology and assembled structure can be well controlled. The luminescent properties of Eu3+ doped Y2O2S with different morphology are studied. The sphere nano-aggregates show the strongest emission intensity because of less surface defects. Under 980 nm laser excitation, Y2O2S:Yb3+, Er3+ nano-phosphors exhibit a tunable emission, shifting from greenish-yellow to orange-yellow, by increasing Yb3+/Er3+ ratio. The solvothermal reaction method reported in this paper is also helpful for the synthesis of other lanthanide oxysulfide NCs.Acknowledgements
This work is supported by Shanghai Science & Technology Committee (12521102501), Shanghai Educational Committee (11ZR1426500), Innovation Program of Shanghai Municipal Education Commission (14ZZ127), PCSIRT(IRT1269), the Program of Shanghai Normal University (DZL124) and Academic Leaders Training Program of Pudong Health Bureau of Shanghai (Grant no. PWRd2011-09).Notes and references
- L. Ozawa and M. Itoh, Chem. Rev., 2003, 103, 3835–3856 CrossRef CAS PubMed.
- D. Liu, C. e. Cui, P. Huang, L. Wang and G. Jiang, J. Alloys Compd., 2014, 583, 530–534 CrossRef CAS PubMed.
- S. Deng, Z. Xue, Y. Liu, B. Lei, Y. Xiao and M. Zheng, J. Alloys Compd., 2012, 542, 207–212 CrossRef CAS PubMed.
- X. Liu, L. Pan, J. Li, K. Yu, Z. Sun and C. Q. Sun, J. Colloid Interface Sci., 2013, 404, 150–154 CrossRef CAS PubMed.
- Y. Song, Y. Huang, L. Zhang, Y. Zheng, N. Guo and H. You, RSC Adv., 2012, 2, 4777–4781 RSC.
- Y. Song, H. You, Y. Huang, M. Yang, Y. Zheng, L. Zhang and N. Guo, Inorg. Chem., 2010, 49, 11499–11504 CrossRef CAS PubMed.
- F. Zhao and S. Gao, J. Mater. Chem., 2008, 18, 949–953 RSC.
- F. Zhao, M. Yuan, W. Zhang and S. Gao, J. Am. Chem. Soc., 2006, 128, 11758–11759 CrossRef CAS PubMed.
- Y. Ding, J. Gu, J. Ke, Y.-W. Zhang and C.-H. Yan, Angew. Chem., Int. Ed., 2011, 50, 12330–12334 CrossRef CAS PubMed.
- Y.-P. Du, Y.-W. Zhang, L.-D. Sun and C.-H. Yan, J. Am. Chem. Soc., 2009, 131, 3162–3163 CrossRef CAS PubMed.
- J. Gu, Y. Ding, J. Ke, Y. Zhang and C. Yan, Chin. J. Chem., 2013, 71, 360–366 CAS.
- J. Liu, H. Luo, P. Liu, L. Han, X. Zheng, B. Xu and X. Yu, Dalton Trans., 2012, 41, 13984–13988 RSC.
- K. Zhang, D. Zhang, J. Liu, K. Ren, H. Luo, Y. Peng, G. Li and X. Yu, CrystEngComm, 2012, 14, 700–707 RSC.
- Q. Dai, H. Song, M. Wang, X. Bai, B. Dong, R. Qin, X. Qu and H. Zhang, J. Phys. Chem. C, 2008, 112, 19399–19404 CAS.
- G. Blasse, Struct. Bonding, 1976, 26, 43–79 CrossRef CAS.
Footnote |
| † Equal contribution as the first author. |
| This journal is © The Royal Society of Chemistry 2014 |



