Colorimetric and fluorescence recognition of tryptophan and histidine using phthalaldehyde based probe: experimental, computational, cell imaging and fish tissue analysis†
Abstract
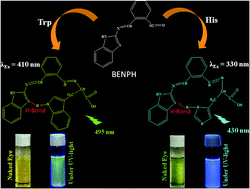
A phthalaldehyde based colorimetric and fluorescence probe (BENPH), selective for tryptophan (Trp) and histidine (His) has been developed. BENPH and its Trp and His adducts are characterized by FTIR, 1H NMR and ESI-Mass spectroscopy. Computational studies support the experimental findings. BENPH can image intracellular Trp and His under a fluorescence microscope. The developed method is used to determine Trp and His in fish muscle.

 Please wait while we load your content...
Please wait while we load your content...