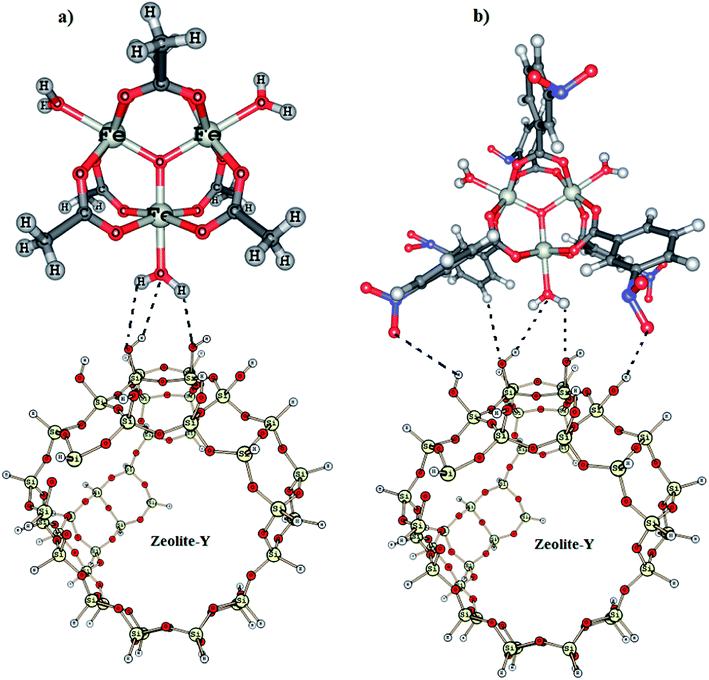
Oxidative coupling of 2-naphthol by zeolite-Y supported homo and heterometallic trinuclear acetate clusters†
Sameeran Kr. Das,
Sanjeev P. Mahanta and
Kusum K. Bania*
Department of Chemical Sciences, Tezpur University, Assam 784028, India. E-mail: kusum@tezu.ernet.in
First published on 3rd October 2014
Abstract
Two trinuclear acetate clusters of iron and cobalt of general formula [Fe3O(O2CCH3)6(H2O)3]NO3·2H2O and Fe2Co(O)[(OOCC6H4NO2)6]NO3·2H2O are synthesized and characterized. The synthesized trinuclear clusters are supported on zeolite-Y via an ion exchanged method. FTIR study reveals that the two complexes are tethered via formation of Si–O–H⋯O–H hydrogen bond linkages with a zeolite-Y matrix. Homogeneous and heterogeneous trinuclear catalysts are found to be efficient catalysts for oxidative coupling of 2-naphthol. Compared to homometallic oxo-clusters, bimetallic complexes are found to show better catalytic activity. Besides obtaining BINOL as a major product, these metal clusters also lead to formation of the tautomeric form of BINOL. The crystal structure of the by-product indicates the formation of a tetrahedral chiral centre in the molecule via the attachment of the solvent. Density Functional Theory (DFT) calculations have been performed to elucidate the structural and electronic properties of both homogeneous and heterogeneous complexes.
1. Introduction
Contemporary interest in the catalytic oxidation of alcohols has led researchers to develop catalysts which could help in the production of fine chemicals.1–3 Out of the various catalytic oxidation processes, oxidation of 2-naphthol to BINOL is among the principal target reactions as both (S)-BINOL and (R)-BINOL are used as chiral auxiliaries in asymmetric synthesis.4–6 Selective oxidation of 2-naphthol to either (S) or (R)-BINOL has been achieved using various transition metal catalysts.7–9 Katsuki and co-worker have reported for asymmetric synthesis of chiral BINOL using chiral iron–Schiff base complexes.10,11 From our group we also account for role of cation–π interaction in enantioselective conversion of 2-naphthol to R-BINOL by zeolite-Y encapsulated Fe–Schiff base complexes.12 Besides the transition metal complexes recently, various bimetallic transition metal oxides are found to be active for such C–C bond coupling reaction.13Although, some homogeneous catalyst has been found to be effective for such reaction using molecular oxygen as oxidant but there are only few heterogeneous catalysts reported for the oxidative biaryl coupling reaction. Cu2+ and Fe3+exchanged exchanged MCM-41,14,15 Fe3+ and Cu2+ exchanged montmorillonite,16,17 and vanadium–Schiff-base/SiO218 are few examples of such heterogeneous catalysts used in the synthesis of biaryl compounds. However, the reported procedures has certain disadvantages in terms of the turnover number, deactivation of catalyst and used of hazardous solvent such as chlorobenzene and chloroform. Matsushita et al.19 reported for a supported ruthenium hydroxide catalyst (Ru(OH)x/Al2O3) as an effective heterogeneous green catalyst for the aerobic biaryl coupling of 2-naphthols and substituted phenols in water without any additives.
With the aim of arriving at a cleaner, more efficient catalytic system for the production of BINOL from 2-naphthol, we have designed a new catalyst that entails harnessing the advantages of oxo-centered trimeric Fe(III) and Co(III) acetates20 and zeolite-Y.21 Oxo-bridged transition-metal carboxylate clusters with auxiliary N- and O-donor ligands are interesting because of their complex and diverse structural motifs.22 These type of metal complexes are also well known due to their optical, catalytic and magnetic properties.23,24 They exhibit very good properties as homogeneous catalysts25 and also act as building blocks for designing of supramolecules.26 In recent years they have been widely used as precursors for synthesis of mixed metal oxide nanoparticles like CoFe2O4, NiFe2O4 and spinel ferrite nanoparticles.27–31
Zeolite-Y with high pore dimension of 7.4 Å is known to act as suitable host and also as support for transition metal complexes.32 Zeolite-Y due to their special shape selectivity property allows a convenient route for encapsulation of transition metal complexes.33 Various different types of transition and organometallic complexes and also transition metal clusters either encapsulated or supported on zeolite-Y are found to be effective heterogeneous catalyst for various chemical reactions.34–36 Recently, we have reported for applicability of zeolite-Y as superior host in preventing the stability and catalytic activity of a chiral Cu–Schiff base complex.37,38 Thus looking at the advantages of trinuclear oxo-centered FeIII/CoIII metal oxalates and zeolite-Y, we immobilized two complexes over zeolite-Y via ion exchanged method and used them as catalysts for catalytic oxidation of 2-naphthol. The supported metal complexes are found to convert naphthol to BINOL with high yield.
2. Experimental and theoretical methods
2.1. Experimental section
3. Results and discussion
3.1. Elemental analysis
In order to confirm the exchange of metal complex with NaY we first estimate the amount of metal content and also C, H and N contents in the two heterogeneous systems. EDX, AAS, and CHN techniques are used for such analyses. Results of the elemental analyses are given in Table 1. EDX study of parent NaY zeolite gives Si/Al ratio of 2.76, which corresponds to a unit cell formula Na52[(AlO2)52(SiO2)140]. This ratio remains same on ion-exchanged with the cationic complexes. The amount of metal contents determined by various techniques as depicted in Table 1 indicates that on ion exchanged there occurs a loss certain amount of metal contents. This is often a usual case in heterogenisation of a homogeneous catalyst. There are several reports observing such kind of metal leaching. C, H and N analysis of the complexes are found to be in agreement with the expected one. This further suggest for the successful exchange of the metal complexes with zeolite-Y.| Sample | EDX-analysis | AAS | CHN-analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Si | Al | Na | M | C | N | Al | Na | M | C | N | H | |
| NaY | 21.4 | 8.5 | 7.5 | — | — | — | 8.59 | 7.58 | — | — | — | |
| Complex 1-Y | 21.4 | 8.2 | 5.6 | (Fe3+), 2.5 | 22.1 | 8.2 | 5.5 | (Fe3+), 2.7 | 24.1 | — | 5.0 | |
| Complex 2-Y | 21.4 | 8.6 | 5.4 | (Fe3+), 8.4 | 40.4 | 6.6 | 8.6 | 5.3 | (Fe3+), 8.7 | 40.5 | 6.7 | 2.9 |
| (Co3+), 4.4 | (Co3+), 4.66 | |||||||||||
3.2. Powder X-ray diffraction, PXRD analysis
PXRD patterns of neat zeolite-Y, zeolite-Y supported iron and iron–cobalt trinuclear oxo acetate cluster are shown in Fig. 1. The XRD pattern of the neat zeolite-Y and those supported with metal clusters are same. However, the XRD peaks are found to get slightly shifted due to formation of the trinuclear metal-oxo clusters. This change in XRD pattern suggest for the surface modification of zeolite-Y due to complexation with metal complexes. | ||
| Fig. 1 PXRD pattern of zeolite-Y (black) and zeolite-Y supported metal complex (red). (c and d) are expanded form of (a and b), respectively. | ||
3.3. Fourier transform infrared spectroscopy (FTIR)
The FTIR spectra of zeolite-Y, neat complexes and zeolite-Y-supported trinuclear oxo-metal complexes are shown in Fig. 2 and 3. IR spectra of zeolite-Y show strong zeolite bands in the region 450–1200 cm−1, Fig. 2a and 3a. The strong band in the region 1000–1100 cm−1 can be attributed to the asymmetric stretching vibration of (Si/Al)O4 units.46 The broad band at the region 1650 and 3500 cm−1 are due to lattice water molecules and surface hydroxylic group, respectively. The IR bands of all zeolite-Y supported complexes are weak due to their low concentration and thus can only absorb in the region 1200 to 1600 cm−1 where the zeolite matrix does not absorb. The characteristics peaks shown in FTIR spectrum for the complexes in Fig. 2 and 3 are assigned in Table 2. It can be observed from Fig. 2b and 3b that both the complexes supported on zeolite-Y possesses the similar characteristics bands present in the neat complexes, Fig. 2c and 3c. However, there occurs some shifting in the vibrational bands due the interaction of the metal complexes with the zeolite matrix.| Compound | Wave number (in cm−1) | Band assignment |
|---|---|---|
| Zeolite-Y | 1647, 3461 | ν(O–H) lattice water, surface hydroxyl |
| 1000 | νas(Si–O/Al–O) | |
| 553, 453 | νs(Si–O/Al–O) | |
| Complex 1 | 3443 | νs(O–H) |
| 2993, 2850 | νs(C–H) | |
| 1747, 1638 | νa(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
|
| 1554 | νa(COO−) | |
| 1408 | νs(COO−) | |
| 1037 | νs(CO) + δ(O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
|
| 663, 608 | Crystal water | |
| 553 | ν(MO) + ν(CC) | |
| Complex 2 | 1619, 1480 | νa(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)aromatic C)aromatic |
| 1531 | νa(COO−) | |
| 1408 | νs(COO−) | |
| 1336 | νs(NO) | |
| 1081–1162 | νs(CO) + δ(O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
|
| 629, 719, 784, 923 | Aromatic ring deformation | |
| 542 | ν(MO) + ν(CC) | |
| 475 | Ring.def + δ(O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
|
| Comple 1-Y | 3467 | νs(O–H) |
| 2928 | νs(C–H) | |
| 1656 | νa(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
|
| 1545 | νa(COO−) | |
| 1419 | νs(COO−) | |
| 1302 | –CH3 bending | |
| Complex 2-Y | 3431 | νs(O–H) |
| 1654 | νa(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
|
| 1553 | νa(COO−) | |
| 1480 | νa(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)aromatic C)aromatic |
|
| 1404 | νs(COO−) | |
| 1352 | νs(NO) | |
| 665 | Aromatic ring deformation |
The most significant change that is observed in the FTIR spectrum of the two supported complexes is in the OH stretching region of zeolite-Y. The IR spectrum of the parent zeolite-Y sample activated at 550 °C displays three bands in the OH stretching region, at 3744, 3724, and 3607 cm−1. According to the literature,36,47 the band at 3744 cm−1 corresponds to external silanols, and that at 3607 cm−1 corresponds to bridging acidic OH groups. The weak band at 3724 cm−1 is attributed to internal silanols. On ion-exchanged with Complex 1 and Complex 2, we observe several new peaks in the region of 3700–4000 cm−1, Fig. 4a and b. In case of Complex 1-Y, we observe peak at 3792, 3850 and 3924 cm−1 in addition to a peak at 3742 cm−1, Fig. 4a. This is obvious because the co-ordinated H2O molecules of Complex 1 may interact with Si–OH groups of zeolite-Y in different ways, Fig. 5a. The presences of 3742 cm−1 however, indicate that all the Si–OH group are not involved in H-bond formation. In case of Complex 2-Y, we observe vibrational bands at 3742, 3754, 3807, 3851, 3895 and 3961 cm−1, Fig. 4b. The appearance different vibrational band indicates that in Complex 2-Y not only H2O but also NO2 group of the aromatic ligand are involved in the formation of H-bond with zeolite-Y, Fig. 5b. The model describe in Fig. 5 is based on our previously reported work.48
3.4. 29Si and 13C NMR analysis
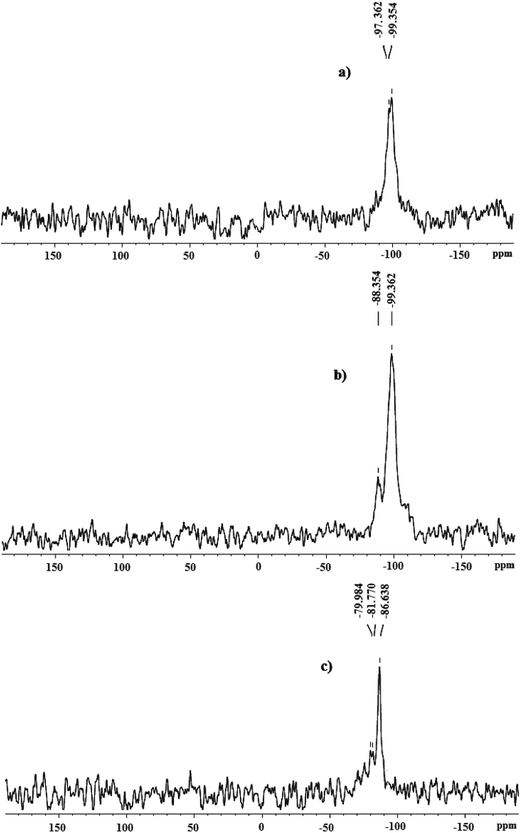
The heterogeneous trinuclear oxo-metal complexes are further characterized by 29Si and 13C NMR spectroscopy. 29Si NMR spectrum of zeolite-Y shows broad peak in between −97.3 to −99.3 ppm, Fig. 6a. However, on complexation with Complex 1, chemical shift values are shifted to −88.3 ppm (Fig. 6b) while on complexation with Complex 2 it shifted to −81.7 ppm, Fig. 6c. The up field shift in the 29Si NMR spectrum of zeolite-Y on modification with Complex 1 and Complex 2 indicates that the chemical environment near Si in zeolite-Y does not remain the same. 13C NMR spectrum of Complex 1-Y (Fig. 7a), shows signal at 179.0 ppm due to carbon atom of the carboxylate group of acetate moiety co-ordinating with iron centres. The peak at 21.7 ppm corresponds to methyl group of acetate ligand, Fig. 7a. This result shows that acetate groups of Complex 1-Y are in similar chemical environment. 13C NMR spectrum of Complex 2-Y shows peak at 169.0, 148.1, 135.1, 131.1, 126.1 and 125.3 ppm corresponding to the aromatic m-nitro benzoic acetate group co-ordinating with the Fe and Co atom of Complex 2, Fig. 7b. Results obtained from the solid state 29Si and 13C NMR analysis confirms the formation of heterogeneous Complex 1-Y and Complex 2-Y.3.5. UV-vis/DRS spectroscopy
The UV-vis spectrums of the neat complexes are recorded in solution phase whereas those of the supported complexes are measured in solid phase in reflectance mode. The UV-vis spectrum of a methanolic solution of Fe-oxo-cluster, Complex 1, shows bands at 211, 313 and 491 nm. The sharp intense band at 211 nm is due to π → π* transition originated from the carboxylate moiety. The bands at 313 and 491 nm are because of the Fe3+ ion and can be assigned to 4T1 → 4T2 and 4T1 → 4A1 transition, (Fig. 8a). In the corresponding, Fe–Co cluster, absorption bands are observed at 217, 310, 468 and 613 nm, (Fig. 8b). The high energy band at 217 nm is due to π → π* transition originated from the aromatic ring of m-nitro benzoic acid. The band at 310 nm is due charge transfer transition (MLCT) from metal d-orbital to the π* orbital of the ligand. The band at 468 nm is for Fe3+ ion (4T1 → 4T2) and the band at 613 nm is because of the ligand field transition originated from Co3+ (1A1 → 1T1).The diffuse reflectance spectrums of the supported metal complexes are depicted in Fig. 9. The DRS spectrum of Complex 1 (Fig. 9a) shows bands at 217 nm and 488 nm due to π → π* and d–d transition originated from the ligand and the metal ion, respectively. The presence of the similar electronic transitions indicates the presence of metal complex on the zeolite-Y surface. DRS spectrum of Complex 2 shows band at 223, 268, 351, 476 and 630 nm, (Fig. 9b). This indicates the formation of metal complex on the surface of zeolite-Y. In both the supported complexes the peaks are found to be slightly red shifted in comparison to the neat complexes due to the effect of the zeolite matrix.
3.6. ESR analysis
The type of trinuclear metal complexes discussed in the present work are characterized by an S = 1/2 ground state with an excited state with S = 3/2 at 25 cm−1.49 ESR spectrum recorded from a powdered sample of Complex 1-Y at 77 K is shows in Fig. 10. At room temperature we do not observe any ESR signal for the Complex 1-Y. At low temperature i.e. at liquid nitrogen temperature (77 K) broad signals are observed with a prominent feature at g ∼ 2.0 for S = 1/2 state. The signal at g ∼ 2.0 consists of an absorption peak at g ∼ 2.12 a derivative feature at g ∼ 2.0 and a valley at 1.89. In addition to this a weak signal observed at g ∼ 4.3 is characteristics of FeIII (S = 5/2) complex. In absence of any inter-trimer interaction a symmetric derivative is expected at g ∼ 2.0 for S = 1/2. However, because of the anisotropic effect may lead to splitting of symmetric line. Thus the splitting of the isotropic g values indicates the presence of weak internuclear interaction. It has been reported that ESR spectra of trinuclear transition metal complexes similar to those of Complex 1 and Complex 2 because of non-Heisenberg interactions and/or single-ion zero-field splitting result in a anisotropic S = 1/2 ground state give rise to ESR signal in the g ∼ 2.0 region.493.7. Cyclic voltammogram
The nature of intrazeolite or extrazeolite complexes that are not apparent from spectroscopic evidences can also be obtained from electrochemical study.50The voltammogram of a neat Complex 1 in solution mode at a scan rate of 0.1 V is shown in Fig. 11a. The quasi-reversible reduction process is due to a FeIII/FeII couple, [Fe3IIIO(O2CR)6(H2O)3] ↔ [Fe2IIIFeIIO(O2CR)6(H2O)3]. It shows the oxidation peak due to FeII → FeIII at −0.075 V and the corresponding reduction peaks at 0.152 V. Besides this it also shows a second reduction wave at a more negative potential at −1.149 V. This wave involves two or more electrons and may correspond to metal centered reduction or to the loss of the central oxygen atoms. The cyclic voltammogram taken at different scan rate indicates a decrease in peak intensity of reduction potential, Fig. 11b. In case of zeolite-Y supported metal the oxidation peak is observed at −0.509 V and the two reduction potentials are observed at −0.219 V and −0.626 V, Fig. 12a. The change in the redox potential values can be attributed to the effect of zeolite matrix. The reduction peak potentials of Complex 1-Y are also found to depend on scan rate, Fig. 12b.
Cyclic voltammogram of Complex 2 shows number of peaks and are found to be scan rate dependent (Fig. 13a and b). Peak I at −1.048 V is due to oxidation of FeII to FeIII, peak II at −0.683 V corresponds to oxidation of CoII to CoIII. III and IV is for reduction of FeIII → FeIII and CoIII → CoII whereas the other two reduction potential wave (V and VI) appearing close to −1.0 V may correspond to metal centered reduction or to the loss of the central oxygen atoms. The redox potential values corresponding to Complex 2-Y are found to differ due to the effect of zeolite matrix, Fig. 14a and Table 3. The electrochemical behaviour of the complexes found to depend on scan rate. The intensity of the reduction peak gets diminished with the decrease of scan rate, Fig. 14b.
| Complexes | E1oxd | E2oxd | E1red | E2red | E3red | E4red | Eavoxd | Eavred |
|---|---|---|---|---|---|---|---|---|
| Complex 1 | −0.075 | — | 0.152 | −149 | −0.075 | −0.498 | ||
| Complex 2 | −0.683 | −1.048 | −076 | −0.385 | −0.911 | −1.261 | −0.865 | −0.658 |
| Complex 1-Y | −0.509 | −0.219 | −0.626 | — | — | −0.509 | −0.422 | |
| Complex 2-Y | −0.203 | — | −0.396 | −0.849 | — | — | −0.203 | −0.622 |
3.8. SEM and TEM analysis
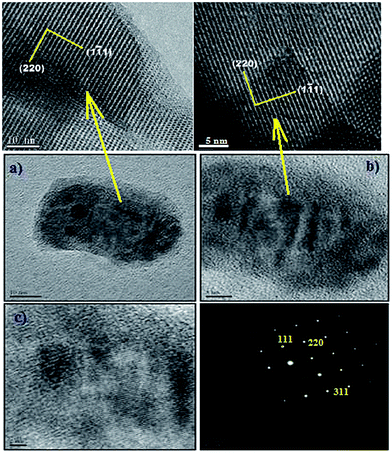
While synthesizing metal complexes in zeolites the complexes may form inside the cavities or on the surface. SEM technique gives an insight whether the complexes are present on the surface of zeolite. Some representative SEM pictures are shown in Fig. 15. Fig. 15 represents a typical morphology of the zeolite surface for all the considered systems and it shows the deposition of complexes or the uncomplexed species on the external surface, Fig. 15b and c. The TEM (Fig. 16) also directly reveals the intact porosity of the support after immobilization of the metal complex. Massive agglomerations of smaller particles are observed on the surface of the zeolite-Y. These agglomerates consist of many small crystallites clustered together (Fig. 16) to form larger aggregates that are distributed on the support surface.3.9. TGA analysis
The TG patterns of parent neat complexes and zeolite-Y supported metal clusters are displayed in Fig. 17. The neat Complex 1 has four weight loss steps at about 32, 114, 196 and 300 °C while the Complex 2 shows three weight loss steps at about 93, 335, 418 °C. On the basis of the weight changes, the first weight-loss step in both neat complexes corresponds to the loss of water molecule as an endothermic phenomenon. The second weight loss at 93–114 °C is due to loss of co-ordinated water molecules. The third weight loss at 196 °C in Complex 1 may be related to the loss of acetate group and that in 300 °C may be due to loss of Fe2O3. There is a sharp weight change at 334 °C case of Complex 2 which is attributed to loss nitro-benzoic acid and that above 418 °C is due to loss of metal oxides. However, for the corresponding immobilized complexes the weight loss extend up to 560 °C. On the basis of thermal analysis data, we may conclude that zeolite-Y supported metal complexes may be treated thermally without any significant decomposition.4. Catalytic activity
4.1. Oxidative coupling of 2-naphthol by neat and zeolite supported metal complexes
The oxidative coupling of 2-naphthol is a representative reaction mode for direct synthesis of 1,1′-binaphthol (BINOL) whose optically pure derivatives are regarded as versatile chiral auxiliaries and ligands in asymmetric synthesis. However, these substances remain largely unstudied because of their difficulties in synthesis. We found that the Fe and Fe–Co cluster complexes tethered via H-bonding in zeolite-Y creates a catalyst that allows highly selective transformation of 2-naphthol to BINOL under aerobic condition without any additive. Catalytic conversion of 2-naphthol to BINOL is effected both by temperature and solvent system. So in order to observe the effect of temperature and solvents, we screened the reaction at different temperature and solvents. For this we consider Complex 1 as test catalyst and perform the reaction in different solvents like methanol, dichloromethane (DCM), dimethylformamide (DMF) and toluene refluxing at 30 °C. Most of the complexes showed very poor or no catalytic activity in these solvents at 30 °C. However, on increasing the temperature up to 45 °C in methanol, the neat complexes give the racemic mixture with moderate yield. Reaction performed at ice cool condition does not show any conversion. Hence, the catalytic reaction by all other three catalysts is monitored at 45 °C in methanol. Interestingly, besides isolating BINOL we also obtained minor product which we crystallized and found to be differ from BINOL. The structure of isolated minor product is shown in Fig. 18. The minor product is a tautomeric form of the BINOL possessing a chiral centre created by the attachment of the solvent moiety. Complex 2 shows high selectivity and gives better yield as compared to the Complex 1. Under the same conditions the catalytic activity of the encapsulated complexes are tested for oxidative coupling of 2-naphthol. It can be seen from the data given in Table 4 that the supported complexes give high percentage yield and selectivity in comparison with the neat complexes. We also observe the effect of catalyst amount on the catalytic process. Up to 10 mg of the catalyst we do not observe any progress of the reaction in TLC test. However, after addition of 15 mg of the catalyst conversion of naphthol to BINOL is observed and maximum conversion is obtained on using 25 mg of the catalyst. On increasing the amount of catalyst no significant enhancement in the catalytic conversion is seen. Therefore, the reaction is performed with 25 mg of the catalysts. Among all the complexes, Complex 2-Y shows good conversion of 2-naphthol to BINOL. Blank experiments carried out without the catalyst under identical experimental conditions do not produce BINOL indicating the participation of the iron and iron–cobalt trinuclear oxo-complexes in the reaction path. The use of stoichiometric amount of complexes as oxidizing agent under nitrogen atmosphere do not yield the product indicating that oxygen is essential for the reaction. The time required for this catalytic conversion is found to be less in case of the encapsulated complexes in comparison to the corresponding neat complexes. Oxidative coupling of 2-naphthol with the neat complexes resulted in the formation of racemic BINOL along with the minor chiral product. Further, we could isolate only 45% to 48% of the desired product in case of the reaction catalysed by Complex 1 and Complex 2. However, oxidative coupling of 2-naphthol with Complex 1-Y and Complex 2-Y under similar conditions results >80%. The catalytic conversion of naphthol to BINOL by Complex 2-Y has also been monitored through UV-vis study. The UV-vis spectrum of 2-naphthol shows its characteristics peak at 290 and 318 nm. However, as the reaction proceeds, we observe two new peaks at 266, 277 and 288 nm characteristic of BINOL (Fig. 19). This further confirms the catalytic activity of the synthesized bimetallic Fe–Co oxo cluster.| Catalysts | Time | (%) Yielda | Fe-atom in catalystb (mmol) | (%) Yield in mmol | TON |
|---|---|---|---|---|---|
| a The reactions were carried out in methanol with catalyst (25 mg) on a 5 mmol scale under aerobic conditions. a Determined by chromatographic separation. b Amount of Fe-atom in mmol present per 25 mg of catalyst. | |||||
| Zeolite-Y | 72 h | Nil | — | Nil | — |
| Complex 1 | 72 h | 45 | 0.0062 | 2.25 | 363 |
| Complex 2 | 56 h | 48 | 0.0020 | 2.4 | 1200 |
| Complex 1-Y | 24 h | 70 | 0.0058 | 3.5 | 603 |
| Complex 2-Y | 18 h | 86 | 0.0018 | 4.3 | 2388 |
4.2. Recyclability test
The homogeneous trinuclear-oxo clusters could not be retrieved after the oxidation process. However, we could successfully recover the zeolite-Y supported metal clusters. To study the recyclability of supported catalyst during oxidation of 2-napthol, we carry out a few reaction under similar condition with Complex 1-Y and Complex 2-Y. After the first run of the oxidation reaction, the catalysts are separated by centrifugation. Fresh reactants are added to the supernatant. Analysis of the reaction mixture shows no further increase in the conversion of 2-napthol. UV-vis study of the supernatant also gives indication for the absence of trace amount of the metal complex. After separation, catalyst are washed thoroughly with petroleum ether, dried, and subjected to another cycle with fresh reactants under similar conditions. The conversion and % yield are found to be consistent. The above procedure is repeated for three cycles, and we do not observed any substantial loss in the catalytic activity of supported catalyst. This indicates that trinuclear metal oxo-clusters are tightly bound to the silanol group of zeolite-Y. However, after third cycle i.e. in fourth and fifth cycles we observed substantial decrease in the catalytic activity. UV analysis of the supernatant indicates leaching of the metal content. The amount of metal loss (Fe) after third and fifth cycle as determined by Vogel's method39 is given in Table 5. Further, cyclic voltammetry analysis of the separated catalyst shows almost similar behavior neat complexes suggesting the detachment of the metal complexes from the solid support thereby perturbing the catalytic activity of the catalyst. Thus, due to metal leaching and rupturing of the H-bond interaction between the complexes and zeolite-Y moiety leads to substantial decrease in the catalytic activity of the heterogeneous catalyst.4.3. Theoretical study
Density Functional Theory calculation (DFT) has been performed on Complex 1 and Complex 1-Y in order to understand the structural and electronic difference between the neat and the encapsulated system. The geometrical parameters such as bond length and bond angles obtained from VWN/DN level calculations for the neat and the encapsulated complexes are found to be in good agreement with the available crystal structure of such complexes.51The bond distances between the oxygen atom of silanol hydroxyl group and hydrogen atom of H2O group ranges from 1.94 to 2.56 Å. These distances are smaller than the sum of the van der Waals radii (2.6 Å) of T-atoms of zeolite and hydrogen atom of water. These results indicate the existence of Si–O⋯H–O hydrogen bond which is in accordance with our experimental FTIR result. The occurrence of H-bond indicates the possibility of tethering of the metal complex over zeolite surface leading to the formation of a supramolecular host–guest complex.
The HOMO and LUMO orbitals corresponding to those of the neat and the encapsulated complexes are shown in Fig. 20. HOMO and LUMO orbitals in neat Complex 1 are concentrated at the metal centre while the same in case of Complex 1-Y are partly distributed over the metal complex and zeolite framework. It can be seen that HOMO orbital in case of Complex 1-Y lies higher in energy than the neat Complex 1. This further reveals the fact since the metal complexes are involved as catalyst in oxidative coupling of 2-naphthol where the rate determining step is the oxidation of FeIII to FeIV by molecular oxygen therefore this oxidation process will be more feasible in case of Complex 1-Y then in comparison to Complex 1.
Acknowledgements
The author thanks Prof. R. C. Deka, Dept. of Chemical Sciences, Tezpur University for helping in computational study. Dr Tridip Sarma, University of Hyderabad for help and suggestion.References
- Y. Zhu, B. Zhao and Y. Shi, Org. Lett., 2013, 15, 992–995 CrossRef CAS PubMed
.
- G. L. H. Tapley, M. J. Silvero, C. J. B. Alejo, M. G. Béjar, C. D. McTiernan, M. Grenier, J. C. N. Ferreira and J. C. Scaiano, J. Phys. Chem. C, 2013, 117, 12279–12288 Search PubMed
.
- J. V. Sundar and V. Subramanian, Org. Lett., 2013, 15, 5920–5923 CrossRef PubMed
.
- J. M. Brunel, Chem. Rev., 2005, 105, 857–897 CrossRef CAS PubMed
.
- Y. Chen, S. Yekta and A. K. Yudin, Chem. Rev., 2003, 103, 3155–3212 CrossRef CAS PubMed
.
- J. Brussee, J. L. G. Groenendijk, J. M. Koppele and A. C. A. Jansen, Tetrahedron, 1985, 41, 3313–3319 CrossRef CAS
.
- J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359–1469 CrossRef CAS PubMed
.
- Q. X. Guo, Z. J. Wu, Z. B. Luo, Q. Z. Liu, J. L. Ye, S. W. Luo, L. F. Cun and L. Z. Gong, J. Am. Chem. Soc., 2007, 129, 13927–13938 CrossRef CAS PubMed
.
- S. E. Allen, R. R. Walvoord, R. P. Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234–6458 CrossRef CAS PubMed
.
- H. Egami, K. Matsumoto, T. Oguma, T. Kunisu and T. Katsuki, J. Am. Chem. Soc., 2010, 132, 13633–13635 CrossRef CAS PubMed
.
- H. Egami and T. Katsuki, J. Am. Chem. Soc., 2009, 131, 6082–6083 CrossRef CAS PubMed
.
- K. K. Bania, D. Bharali, B. Viswanathan and R. C. Deka, Inorg. Chem., 2012, 51, 1657–1674 CrossRef CAS PubMed
.
- M. V. Maphoru, J. Heveling and S. K. Pillai, ChemPlusChem, 2014, 79, 99–106 CrossRef CAS
.
- M. R. Prasad, G. Kamalakar, S. J. Kulkarni and K. V. Raghavan, J. Mol. Catal. A: Chem., 2002, 180, 109–123 CrossRef
.
- E. Armengol, A. Corma, H. García and J. Primo, Eur. J. Org. Chem., 1999, 1915–1920 CrossRef CAS
.
- M. L. Kantam, B. Kavita and F. Figueras, Catal. Lett., 1998, 51, 113–115 CrossRef CAS
.
- P. Mastrorilli, F. Muscio, G. P. Suranna, C. F. Nobile and M. Latronico, J. Mol. Catal. A: Chem., 2001, 165, 81–87 CrossRef CAS
.
- M. Tada, T. Taniike, L. M. Kantam and Y. Iwasawa, Chem. Commun., 2004, 2542 RSC
.
- M. Matsushita, K. Kamata, K. Yamaguchi and N. Mizuno, J. Am. Chem. Soc., 2005, 127, 6632–6640 CrossRef CAS PubMed
.
- R. C. Mehrotra and R. Bohra, Metal Carboxylates, Academic Press, London, 1983 Search PubMed
.
- M. Heitbaum, F. Glorius and I. Escher, Angew. Chem., Int. Ed., 2006, 45, 4732–4762 CrossRef CAS PubMed
.
- C. D. Chandler, C. Roger and M. J. Hampden-Smith, Chem. Rev., 1993, 93, 1205–1220 CrossRef CAS
.
- G. Losada, M. A. Mendiola and M. T. Sevilla, Inorg. Chim. Acta, 1997, 255, 125–131 CrossRef CAS
.
- H. Vrubel, T. Hasegawa, E. de Oliveira and F. S. Nunes, Inorg. Chem. Commun., 2006, 9, 208–211 CrossRef CAS PubMed
.
- S. Murata, M. Miura and M. Nomura, J. Chem. Soc., Perkin Trans. 2, 1989, 617–621 RSC
.
- H. E. Toma, K. Araki, A. D. P. Alexiou, S. Nikolau and S. Dovidauskas, Coord. Chem. Rev., 2001, 219–221, 187–234 CrossRef CAS
.
- Y. Yuan, L. Chen, R. Yang, X. Lu, H. Peng and Z. Luo, Mater. Lett., 2012, 71, 123–126 CrossRef CAS PubMed
.
- L. Chen, Y. Yuan, H. Peng, X. Lu, and Z. Luo, Mater. Lett., 2012, 67, 311–314 Search PubMed.
- L. Chen, Y. Shen and J. Bai, Mater. Lett., 2009, 63, 1099–1101 CrossRef CAS PubMed
.
- K. P. Naidek, F. Bianconi, T. Costa, R. da Rocha, D. Zanchet, J. A. Bonacin, M. A. Novak, M. d. G. F. Vaz and H. Winnischofer, J. Colloid Interface Sci., 2011, 358, 39–46 CrossRef CAS PubMed
.
- K. O. Abdulwahab, M. A. Malik, P. O'Brien, G. A. Timco, F. Tuna, R. E. P. Winpenny, R. A. D. Pattrick, V. S. Cokerd and E. Arenholze, J. Mater. Chem. C, 2014, 2, 6781–6789 RSC
.
- K. K. Bania and R. C. Deka, J. Phys. Chem. C, 2011, 115, 9601–9607 CAS
.
- A. Corma and H. Garcia, Eur. J. Inorg. Chem., 2004, 1143–1164 CrossRef CAS
.
- D. J. Xuereb and R. Raja, Catal. Sci. Technol., 2011, 1, 517–534 CAS
.
- M. Stratakis and G. Froudakis, Org. Lett., 2000, 2, 1369–1372 CrossRef CAS PubMed
.
- K. K. Bania and R. C. Deka, J. Phys. Chem. C, 2013, 117, 11663–11678 CAS
.
- K. K. Bania, G. V. Karunakar, K. Goutham and R. C. Deka, Inorg. Chem., 2013, 52, 8017–8029 CrossRef CAS PubMed
.
- K. K. Bania, G. V. Karunakar, B. Sarma and R. C. Deka, ChemPlusChem, 2014, 79, 427–438 CrossRef CAS
.
- A. I. Vogel, Textbook of Quantitative Chemical Analysis, Pearson Education Asia, 6th edn, 2002, p. 394 Search PubMed
.
- H. G. Hecht, in Modern Aspect of Reflectance Spectroscopy, ed. W. W. Wendlandt, Plenum Press, New York, 1968 Search PubMed
.
- SAINT Plus, Bruker AXS Inc., Madison, WI, 2008 Search PubMed
.
- BRUKER AXS (v 6.14), Bruker AXS Inc., Madison, WI, 2008, Search PubMed
.
- A. L. Spek, PLATON, A Multipurpose Crystallographic Tool, Utrecht University, Utrecht, Netherland, 2002 Search PubMed
.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS
.
- B. Delly, J. Chem. Phys., 1990, 92, 508–514 CrossRef PubMed
.
- A. Zecchina and C. O. Arean, Chem. Soc. Rev., 1996, 25, 187–196 RSC
.
- K. Chakarova and K. Hadjiivanov, J. Phys. Chem. C, 2011, 115, 4806–4817 CAS
.
- K. K. Bania and R. C. Deka, J. Phys. Chem. C, 2012, 116, 14295–14310 CAS
.
- A. K. Boudalis, Y. Sanakis, C. P. Raptopoulou, A. Terzis, J. P. Tuchagues and S. P. Perlepes, Polyhedron, 2005, 24, 1540–1548 CrossRef CAS PubMed
.
- V. Ganesan and R. Ramaraj, Langmuir, 1998, 14, 2497–2501 CrossRef CAS
.
- K. I. Turte, S. G. Shova, V. M. Meriacre, M. Gdaniec, A. Yu, J. Lipkowski, J. Bartolome, F. Wagner and G. Filoti, J. Struct. Chem., 2002, 43, 108–117 CrossRef CAS
.
Footnote |
| † CCDC 1015210. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra10103k |
| This journal is © The Royal Society of Chemistry 2014 |