A TiO2 nanofiber/activated carbon composite as a novel effective electrode material for capacitive deionization of brackish water†
Abstract
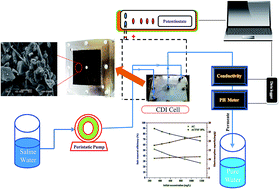
Based on its excellent characteristics, high surface area, environmentally safe and availability in nature; activated carbon (AC) is the best candidate as a capacitive deionization (CDI) electrode material. Among the various modification methods for AC, incorporation of TiO2 nanoparticles into the activated carbon proved to be an effective approach to enhance the desalination performance. Compared to the nanoparticulate morphology, nanofibers have a large axial ratio which provides a better electrosorption performance. Herein, for the first time, TiO2 nanofibers (TNFs) prepared by the electrospinning process were exploited with activated carbon to form a hybrid network electrode for capacitive deionization. The phase morphology and crystal structure were characterized by scanning electron microscopy (SEM) and X-ray diffraction (XRD), respectively. The electrochemical behavior was evaluated by cyclic voltammetry (CV). Furthermore, the desalination performances under different applied voltages and salty water concentrations were investigated using a prototype CDI unit in a continuous mode water flow. The results indicated that the electrode prepared from 10 wt% nanofibers (ACTNF 10%) exhibits the best results compared to other electrodes having 5 and 15 wt% TiO2 nanofibers. Typically, the best electrode networks showed very good specific capacitance (380 F g−1), excellent electrosorptive capacity (17.7 mg per gcarbon) at a cell potential of 1.2 V and an initial NaCl concentration of 292 mg L−1, and a distinguished salt removal efficiency (∼89.6%). Moreover, the introduced electrode shows interesting recyclability and easy full regeneration.

 Please wait while we load your content...
Please wait while we load your content...