Selective interactions of 5-(hydroxyimino)quinolin-8-one with tetrabutylammonium fluoride and zinc(ii) ions†
Abstract
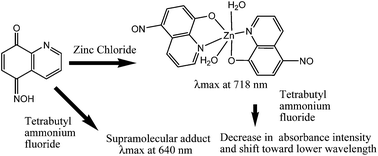
5-(Hydroxyimino)quinolin-8-one (HL) selectively interacts with tetrabutylammonium fluoride to form a supramolecular adduct. Formation of the supramolecular adduct in solution causes a drastic color change or causes quenching of the fluorescence emission of HL enabling its distinction from other tetrabutylammonium halides. Among different metal ions, addition of zinc ions to a solution of HL causes a selective color change or very distinguishable fluorescence emission intensity enhancement from other cations. The selective colouration of HL due to an increase in absorption at ∼640 nm in acidic condition in a similar way upon addition of zinc or fluoride ions is attributed to their ability to deprotonate HL. Di-aqua-bis(5-nitroso-8-oxyquinolinato)zinc(II) is structurally characterized. It shows strong visible absorption at 718 nm, which differs from the absorption of species formed in acidic conditions by zinc causing visual color change.

 Please wait while we load your content...
Please wait while we load your content...