Iodine-catalysed sp3 C–H sulfonylation to form β-dicarbonyl sulfones with sodium sulfinates†
Wen-Chao Gao*,
Jin-Jin Zhao,
Hong-Hong Chang*,
Xing Li,
Qiang Liu and
Wen-Long Wei
College of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan, 030024, P. R. China. E-mail: gaowenchao@tyut.edu.cn; changhonghong@tyut.edu.cn; Tel: +86 0351 6018534
First published on 30th September 2014
Abstract
An efficient and easily handled method for β-dicarbonyl sulfones with sodium sulfinates as the sulfonyl source was developed. This transformation was involved in the iodine-catalysed sp3 C–H sulfonylation of β-dicarbonyl compounds.
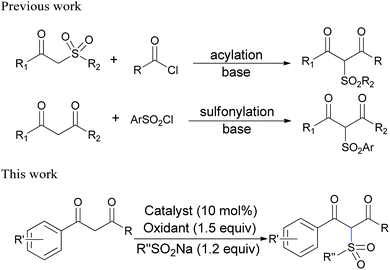
Sulfones belong to a known class of organosulfur compounds, which have found diverse applications in organic synthesis, polymer materials, and medicinal chemistry.1 Among them, β-dicarbonyl sulfones have attracted much attention due to their excellent biological effects, such as antimicrobial,2a anticoagulant2b and anti-schistosomal activities.2c Furthermore, since β-dicarbonyl compounds are commonly used intermediates for heterocycle synthesis,3 β-dicarbonyl sulfones would provide alternative units to construct sulfonylated heteroaromatic compounds in the design of potential drugs.4 Surprisingly, only a limited number of procedures was developed for the synthesis of β-dicarbonyl sulfones during last decades. In most cases, β-dicarbonyl sulfones were prepared through either C-acylation of β-keto sulfones with acyl halides or C-sulfonylation of β-dicarbonyl compounds with sulfonyl halides. These methods usually required excess amount of strong bases (NaOMe,2a,c NaH,5 or LDA6), which are not suitable for sensitive substrates; the acyl or sulfonyl reagents are much reactive and moisture-sensitive, resulting in side reactions and byproducts, especially in the synthesis of complex molecules. Therefore, it is highly desirable to develop an efficient and easily handled method for β-dicarbonyl sulfones with less reactive sulfonyl sources (Scheme 1).
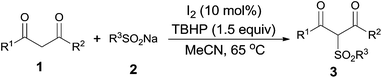
Recently, iodine or tetrabutylammonium iodide has emerged as a promising alternative to catalyse oxidative sulfonylation due to their high efficiency, mild reaction conditions and metal-free features. Especially, the sulfonylation of heteroaromatic compounds and C–C unsaturated bonds has been well established, and examples include regioselective 2-sulfonylation of indoles with sodium sulfinates,7 synthesis of sulfonated pyrazoles with sulfonyl hydrazides,8 sulfonylation of alkenes with sulfonyl hydrazides to form alkenyl sulfones,9a allylic sulfones,9b,c and sulfonated oxindoles,9d and sulfonylation of alkynes with sulfonyl hydrazides to synthesize β-iodovinyl sulfones.9e Although these studies achieved much progress, little attention has been paid to investigate the sulfonylation of sp3 C–H bond. In this regard, we described a novel and efficient method for the synthesis of β-dicarbonyl sulfones by iodine-catalysed sulfonylation of sp3 C–H bond with sodium sulfinates.
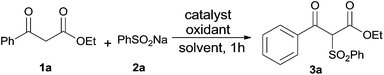
Initially, ethyl benzoyl acetate (1a) and sodium benzenesulfinate (2a) were selected as model substrates to explore the optimal reaction conditions. It was found that ethyl α-phenylsulfonylbenzoylacetate (3a) was obtained in 27% yield by using iodine (10 mol%) and tert-butyl hydroperoxide (TBHP, 1.5 equiv.) in CH3CN at 25 °C (Table 1, entry 1). The yield of 3a could be increased when the reaction temperature was raised (entries 1–3), and the best result (94% yield of 3a) was obtained when heating the reaction mixture to 65 °C (entry 3), while a higher temperature could not give a better result (entry 4). 3a was obtained in slightly lower yields when the reaction was run under N2 atmosphere (entry 5). Control experiments indicated that the desired product 3a could not be determined in the absence of iodine catalyst (entry 6), and only 21% yield of 3a was obtained even using stoichiometric amount of iodine in the absence of TBHP (entry 7). Other catalysts such as KI, Bu4NI, and KIO3 were examined but found less effective than iodine: for KI and Bu4NI, 3a was just afforded in 85% and 66% yields separately (entries 8 and 9); while no desired product was detected when using potassium iodate as the catalyst (entry 10). Two commonly used oxidants (H2O2 and Oxone) were also tested for this transformation, while 3a was produced in inferior yields (entries 11 and 12). Other different solvents were also attempted for this transformation but they failed to provide a more favorable outcome (entries 13–16). For example, 3a could be produced in high yield in the solvents like THF and EtOAc (entries 13 and 14), while low yields of 3a were obtained when using CHCl3 or AcOH as the solvent (entries 15 and 16).
| Entry | Catalyst | Oxidant | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 0.5 mmol of 1a, 0.6 mmol of 2a, 0.05 mmol of catalyst, 0.75 mmol of oxidant, in 2 mL of solvent for 1 h.b Isolated yield.c The reaction was run under N2.d The reaction was run with 1.0 equiv. of iodine. | |||||
| 1 | I2 | TBHP | CH3CN | 25 | 27 |
| 2 | I2 | TBHP | CH3CN | 45 | 73 |
| 3 | I2 | TBHP | CH3CN | 65 | 94 |
| 4 | I2 | TBHP | CH3CN | Reflux | 94 |
| 5c | I2 | TBHP | CH3CN | 65 | 89 |
| 6 | — | TBHP | CH3CN | 65 | 0 |
| 7d | I2 | — | CH3CN | 65 | 21 |
| 8 | KI | TBHP | CH3CN | 65 | 85 |
| 9 | Bu4NI | TBHP | CH3CN | 65 | 66 |
| 10 | KIO3 | TBHP | CH3CN | 65 | 0 |
| 11 | I2 | H2O2 | CH3CN | 65 | 49 |
| 12 | I2 | Oxone | CH3CN | 65 | 17 |
| 13 | I2 | TBHP | THF | 65 | 87 |
| 14 | I2 | TBHP | EtOAc | 65 | 85 |
| 15 | I2 | TBHP | CHCl3 | 65 | 38 |
| 16 | I2 | TBHP | CH3COOH | 65 | Trace |
With the optimal reaction conditions in hand (Table 1, entry 3), a series of β-dicarbonyl compounds (1) was then investigated to couple with sodium benzenesulfinate (2a). It was found that various β-dicarbonyl compounds including β-keto esters, β-diesters and β-diketones were suitable for this transformation (Table 2). Ethyl benzoylacetate derivatives bearing electro-donating or electron-withdrawing group on the phenyl ring gave the corresponding products in good to high yields (3b–d). Other aromatic rings such as naphthyl, furyl, and thienyl groups could also be tolerated, and delivered the corresponding products 3e–g in good to excellent yields. It was noteworthy that the location of a methyl group at the α-position of β-keto esters impeded the reaction process, and the desired product 3h was only furnished in 9% yield. Furthermore, the aliphatic β-keto esters were also attempted and gave the corresponding products in moderate yields (3i, 3j). As for the different β-diesters, the reaction also proceeded well, and gave the desired products 3k, 3l and 3m in moderate yields. The β-diketone such as dibenzoylmethane was proved to be a good substrate for this transformation, and the product 3n was obtained in 81% yield.
| Entry | β-Dicarbonyl compound | Product | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 0.5 mmol of 1, 0.6 mmol of 2a, 0.05 mmol of I2, 0.75 mmol of TBHP (70% in water), in 2 mL of MeCN at 65 °C, for 1–4 h.b Isolated yield.c Conversion: 38%.d Solvent: THF (2 mL). | |||
| 1 | R1 = C6H5, R2 = OEt, R3 = H | 3a | 94 |
| 2 | R1 = 4-BrC6H4, R2 = OEt, R3 = H | 3b | 87 |
| 3 | R1 = 2-MeC6H4, R2 = OEt, R3 = H | 3c | 71 |
| 4 | R1 = 4-MeOC6H4, R2 = OEt, R3 = H | 3d | 87 |
| 5 | R1 = 2-naphthyl, R2 = OEt, R3 = H | 3e | 93 |
| 6 | R1 = 2-furyl, R2 = OEt, R3 = H | 3f | 71 |
| 7 | R1 = 2-thienyl, R2 = OEt, R3 = H | 3g | 82 |
| 8c | R1 = C6H5, R2 = OEt, R3 = Me | 3h | 9 |
| 9 | R1 = iPr, R2 = OEt, R3 = H | 3i | 66 |
| 10 | R1 = tBu, R2 = OEt, R3 = H | 3j | 51 |
| 11 | R1 = CO2Me, R2 = CO2Me, R3 = H | 3k | 66 |
| 12 | R1 = CO2Et, R2 = CO2Et, R3 = H | 3l | 54 |
| 13 | R1 = CO2tBu, R2 = CO2tBu, R3 = H | 3m | 48 |
| 14d | R1 = C6H5, R2 = C6H5, R3 = H | 3n | 81 |
The different sodium sulfinates were also evaluated for this transformation (Table 3). Arylsulfinic acid sodium salts bearing electro-donating or electro-withdrawing substituents on the phenyl ring could smoothly react with ethyl benzoylacetate to give the corresponding products in high yields (3o, 3p). Furthermore, the aliphatic sulfinic acid sodium salts like sodium methanesulfinate were also suitable for this reaction, and coupled with β-keto esters or β-diketones in moderate yields (3q, 3r). Besides, the reactions of β-diketones with different kinds of aromatic sulfinic acid sodium salts also proceeded smoothly, and provided the β-diketo sulfones in high to excellent yields (3s–u).
| Entry | Sodium sulfinate | Product | Yieldb (%) | |
|---|---|---|---|---|
| a Reaction conditions: 0.5 mmol of 1, 0.6 mmol of 2, 0.05 mmol of I2, 0.75 mmol of TBHP (70% in water), in 2 mL of MeCN at 65 °C, for 1–4 h.b Isolated yield.c Solvent: THF (2 mL). | ||||
| 1 | 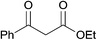 |
R3 = 4-OMeC6H4 | 3o | 85 |
| 2 | R3 = 4-BrC6H4 | 3p | 89 | |
| 3 | R3 = Me | 3q | 43 | |
| 4c |  |
R3 = Me | 3r | 57 |
| 5c | R3 = 4-FC6H4 | 3s | 82 | |
| 6c | R3 = 2-naphthyl | 3t | 89 | |
| 7c | R3 = 4-MeC6H4 | 3u | 95 | |
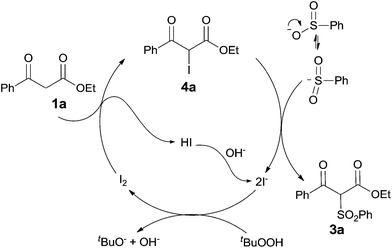
The mechanism of the present transformation is worth discussing. Several control experiments were carried out in order to obtain some insight of the possible mechanism. In the reaction of ethyl benzoylacetate with sodium benzenesulfinate, the α-iodinated ester 4a was detected at the first few minutes. 4a could be isolated in 81% yield in the absence of sodium sulfinates under the standard conditions (Scheme 2a). The treatment of 4a with 1.2 equiv. of PhSO2Na gave the desired product 3a in 90% yield (Scheme 2b). Since another iodinated intermediate benesulfonyl iodide was also likely to be involved under the present conditions,10 the reaction between ethyl benzoylacetate and benesulfonyl iodide was consequently tested, however, only trace amount of 3a was obtained (Scheme 2c).
Based on the results of control experiments and literature reports,11 a plausible mechanism is proposed in Scheme 3. The α-iodination of ethyl benzoylacetate proceeds smoothly under the standard conditions to produce the intermediate 4a. The oxygen-centered anion of sodium benzenesulfinate can be resonated to a sulfonyl anion, of which nucleophilic attraction to the iodinated carbon of 4a would afford the desired product 3a. All released iodide ions can be reoxidized to molecular iodine by TBHP.
In summary, we have developed a novel method for the synthesis of β-carbonyl sulfones with sodium sulfinate as the sulfonyl source under metal-free conditions. This transformation was catalysed by molecule iodine through the sulfonylation of sp3 C–H bond, and the α-iodinated β-dicarbonyl compounds were believed as the key intermediates. The ready availability of starting materials, broad substrate scope, high efficiency and operational simplicity make the present method attractive to construct β-dicarbonyl sulfones and the derived biologically active molecules.
Acknowledgements
We gratefully acknowledge the Natural Science Foundation of Shanxi Province (2012021007-2 and 2011011010-2) and the Qualified Personnel Foundation of Taiyuan University of Technology (no. tyut-rc201307a).Notes and references
- (a) N. S. Simpkins, Sulfones in organic synthesis, in Tetrahedron Organic Chemistry Series, ed. J. E. Baldwin and P. D Margnus, Pergamon Press, Oxford, 1993, vol. 10 Search PubMed; (b) K. Shanak, Synthesis of Sulfones, Sulfoxides and Cyclic Sulfides, ed. S. Patai and Z. Rappoprt, Wiley, Chichester, 1994 Search PubMed; (c) Y. Huang, L. Huo, S. Zhang, X. Guo, C. C. Han, Y. Li and J. Hou, Chem. Commun., 2011, 47, 8904–8906 RSC.
- (a) Y. C. Joshi, S. Saingar, K. P. Joshi and R. Kumar, J. Korean Chem. Soc., 2011, 55, 638–643 CrossRef CAS; (b) M. Jeyachandran and P. Ramesh, Org. Chem. Int., 2011, 360810 Search PubMed; (c) A. F. Eweas, G. Allam, A. S. A. Abuelsaad, A. H. ALGhamdi and I. A. Maghrabi, Bioorg. Chem., 2013, 46, 17–25 CrossRef CAS PubMed.
- (a) S. Mataka, K. Takahashi, Y. Tsuda and M. Tashiro, Synthesis, 1982, 157–159 CrossRef CAS; (b) R. H. Wiley and P. E. Hexner, Org. Synth., 1951, 31, 43 CrossRef CAS; (c) R. K. Saini, Y. C. Joshi and P. Joshi, Heterocycl. Commun., 2007, 13, 219–222 CrossRef CAS.
- (a) X. Chen, S. Hussain, S. Parveen, S. Zhang, Y. Yang and C. Zhu, Curr. Med. Chem., 2012, 19, 3578–3604 CrossRef CAS; (b) G. L. Regina, A. Coluccia, A. Brancale, F. Piscitelli, V. Gatti, G. Maga, A. Samuele, C. Pannecouque, D. Schols, J. Balzarini, E. Novellino and R. Silvestri, J. Med. Chem., 2011, 54, 1587–1598 CrossRef PubMed; (c) A. V. Ivachtchenko, E. S. Golovina, M. G. Kadieva, V. M. Kysil, O. D. Mitkin, S. E. Tkachenko and I. M. Okun, J. Med. Chem., 2011, 54, 8161–8173 CrossRef CAS PubMed.
- Y. Takeuchi, H. Ogura, A. Kanada and T. Koizumi, J. Org. Chem., 1992, 57, 2196–2199 CrossRef CAS.
- Y. K. Yee, P. R. Bernstein, E. J. Adams, F. J. Brown, L. A. Cronk, K. C. Hebbel, E. P. Vacek, R. D. Krell and D. W. Snyder, J. Med. Chem., 1990, 33, 2437–2451 CrossRef CAS.
- F. Xiao, H. Chen, H. Xie, S. Chen, L. Yang and G.-J. Deng, Org. Lett., 2014, 16, 50–53 CrossRef CAS PubMed.
- J. Zhang, Y. Shao, H. Wang, Q. Luo, J. Chen, D. Xu and X. Wan, Org. Lett., 2014, 16, 3312–3315 CrossRef CAS PubMed.
- (a) S. Tang, Y. Wu, W. Liao, R. Bai, C. Liu and A. Lei, Chem. Commun., 2014, 50, 4496–4499 RSC; (b) X. Li, X. Xu and Y. Tang, Org. Biomol. Chem., 2013, 11, 1739–1742 RSC; (c) X. Li, X. Xu and C. Zhou, Chem. Commun., 2012, 48, 12240–12242 RSC; (d) X. Li, X. Xu, P. Hu, X. Xiao and C. Zhou, J. Org. Chem., 2013, 78, 7343–7348 CrossRef CAS PubMed; (e) X. Li, X. Xu and X. Shi, Tetrahedron Lett., 2013, 54, 3071–3074 CrossRef CAS PubMed.
- C. Najera, B. Baldo and M. Yus, J. Chem. Soc., Perkin Trans. 1, 1988, 1029–1032 RSC.
- N. Samakkanad, P. Katrun, T. Techajaroonjit, S. Hlekhlai, M. Pohmakotr, V. Reutrakul, T. Jaipetch, D. Soorukram and C. Kuhakarn, Synthesis, 2012, 1693–1699 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental procedures and characterization data of products. See DOI: 10.1039/c4ra09821h |
| This journal is © The Royal Society of Chemistry 2014 |