SnO2 decorated graphene nanocomposite anode materials prepared via an up-scalable wet-mechanochemical process for sodium ion batteries†
Abstract
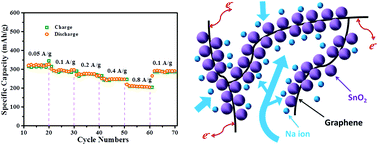
A facile and up-scalable wet-mechanochemical process is designed for fabricating ultra-fine SnO2 nanoparticles anchored on graphene networks for use as anode materials for sodium ion batteries. A hierarchical structure of the SnO2@graphene composite is obtained from the process. The resultant rechargeable SIBs achieved high rate capability and good cycling stability.

 Please wait while we load your content...
Please wait while we load your content...