Multifunctional microparticles with uniform magnetic coatings and tunable surface chemistry†
Tobias P. Niebela,
Florian J. Heiligtagb,
Jessica Kindc,
Michele Zaninia,
Alessandro Lauriab,
Markus Niederbergerb and
André R. Studart*a
aComplex Materials, Department of Materials, ETH Zürich, 8093 Zürich, Switzerland. E-mail: andre.studart@mat.ethz.ch
bLaboratory for Multifunctional Materials, Department of Materials, ETH Zürich, 8093 Zürich, Switzerland
cLaboratory of Metal Physics and Technology, Department of Materials, ETH Zürich, 8093 Zürich, Switzerland
First published on 11th November 2014
Abstract
Microplatelets and fibers that can be manipulated using external magnetic fields find potential applications as miniaturized probes, micromirrors in optical switches, remotely actuated micromixers and tunable reinforcements in composite materials. Controlling the surface chemistry of such microparticles is often crucial to enable full exploitation of their mechanical, optical and sensorial functions. Here, we report a simple and versatile procedure to directly magnetize and chemically modify the surface of inorganic microplatelets and polymer fibers of inherently non-magnetic compositions. As opposed to other magnetization approaches, the proposed non-aqueous sol–gel route enables the formation of a dense and homogeneous coating of superparamagnetic iron oxide nanoparticles (SPIONs) on the surface of the microparticles. Such coating provides a suitable platform for the direct chemical functionalization of the microparticles using catechol-based ligands displaying high affinity towards iron oxide surfaces. By adsorbing for example nitrodopamine palmitate (ND-PA) on the surface of hydrophilic magnetite-coated alumina platelets (Fe3O4@Al2O3) we can render them sufficiently surface active to generate magnetically responsive Pickering emulsions. We also show that microplatelets and fibers coated with a uniform iron oxide layer can be easily manipulated using low magnetic fields despite their intrinsic non-magnetic nature. These examples illustrate the potential of the proposed approach in generating functional, magnetically responsive microprobes and building blocks for several emerging applications.
Introduction
Magnetically responsive particles have been extensively exploited in a variety of fields ranging from sensing,1–3 micro-robotics4,5 and micromanipulation6–8 to environmental9 and bio-medical10–12 applications. When suspended in a non-magnetic fluid, magnetic particles generate a mismatch in magnetic susceptibility between dispersed and continuous phases that can be utilized to control the spatial distribution and orientation of anisotropic particles using external magnetic fields.13–17 While this concept is a straightforward phenomenon for intrinsically magnetic particles, it can also be extended to non-magnetic particles by decorating their surface with superparamagnetic iron oxide nanoparticles (SPIONs).18–20 With this approach, it is possible to magnetically manipulate and align non-magnetic anisotropic platelets and rods with a variety of chemical compositions.20–22 By combining the control over the particle orientation with methods to consolidate the surrounding continuous phase, this technique has been used to fabricate materials with anisotropic porosity,23 hydrogels with programmable shape-changing effects24 and bio-inspired composites with superior mechanical properties.20,25The decoration of non-magnetic particles with iron oxide is usually performed by introducing the microparticles in a suspension of SPIONs, also known as a ferrofluid. Another method to magnetize particles is the in situ synthesis of iron oxide nanoparticles. For example, an aqueous sol–gel reaction was recently used to coat cellulose nanofibrils, thus leading to functional magnetic aerogels.27 However, these methods usually do not allow for the creation of a dense and homogeneous iron oxide coating on the particle surface and are often limited to microparticles that carry electrical charges and are compatible with water. Recently, an emulsion comprising droplets of aqueous ferrofluid suspended in an oil phase was proposed as an alternative vehicle to coat water-soluble calcium sulphate rods with SPIONs.23 Although effective, this procedure also offers limited control over the distribution and density of SPIONs on the microparticle surface.
In addition to magnetic control, the microparticles used in many of the applications mentioned above should also exhibit tailored surface chemistry to fulfill the designed end function. For example, microplatelets might require the attachment of fluorescent molecules on the platelet surface for potential applications in optical devices or sensors.26 In reinforced composite materials, mechanical load transfer from the matrix to the reinforcing microparticle also depends on proper design of the microparticle surface chemistry.28–31 Finally, magnetically responsive fibers and platelets with tunable surface hydrophobicity may enable the preparation of smart Pickering foams and emulsions.32 Given all these potential applications, there is a need for the development of straightforward methods that simultaneously enables magnetic control and surface modification of non-magnetic microparticles.
In this paper, we report a simple and versatile approach to magnetize the surface of microparticles with a dense and homogeneous layer of SPIONs, which allows for direct functionalization of the resulting particles with catechol-based ligands. The non-aqueous nature of the proposed method also renders it more universal than previous routes that rely on electrostatic interactions for surface magnetization. The use of nitrodopamine ligands that can directly bind to iron oxide surfaces offers a straightforward method to equip the magnetized particles with a variety of chemical functionalities.
Experimental section
Materials and reagents
Alumina platelets with an average diameter of 7.5 μm and thickness of 200 nm were provided by Antaria Ltd. (Alusion™, Australia), whereas poly(vinyl alcohol) (PVA) fibres were acquired from STW (F PVA 401/100*, Germany), both were used as received. 10 μm thick aramid filaments from Teijin Aramid BV (Twaron 1060 dtex, Netherlands) were cut into 1 to 3 mm long pieces with a razor blade. The following reagent-grade chemicals were purchased and used as received unless otherwise stated: nitric acid (HNO3, 65%) and potassium nitrate (KNO3, pro analysi) from Merck (Germany), potassium hydroxide (KOH, pro analysi) from Fisher Chemicals (UK), benzyl alcohol (BnOH, 99%) from Acros Organics (Belgium), iron(III) acetylacetonate (Fe(acac)3, 97% and 99.9%), benzyl alcohol (BnOH, anhydrous, 99.8%), ethanol (EtOH) and octane from Sigma-Aldrich (Switzerland).Iron oxide coating of microparticles
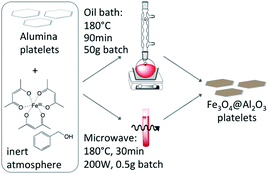
To coat microparticles with SPIONs via the proposed non-aqueous sol–gel route we employed either an oil bath or microwaves as heat source.To coat alumina platelets with iron oxide by heating in the microwave, we typically combined inside an argon filled glove box 0.5 g Alusion platelets, 0.1 g Fe(acac)3 (99.9%) and 5 ml BnOH (99.8%) in a microwave tube equipped with a stir bar and sealed it with a Teflon cap. The resulting suspension was sonicated for three minutes in an ultrasonic bath and heated to 180 °C in a microwave reactor (CEM Discover, USA) for 30 minutes with a maximum irradiation of 200 W. Reference samples of iron oxide nanoparticles for XRD measurements were prepared by the procedure described above without the addition of alumina platelets.
In a typical procedure for coating alumina platelets by heating in an oil bath, 50 g Alusion platelets, 10 g Fe(acac)3 (97%) and 340 ml BnOH (99%) were combined in a 500 ml three neck round bottom flask. After sonicating the suspension for 20 min with a pulse–pause sequence of 1 s–1 s and a relative intensity of 55% using a Vibra-Cell VCX 130 probe sonicator (Sonics & Materials, USA), the flask was equipped with a condenser connected to a one-way gas release and sealed with rubber septae. Nitrogen gas was passed through the mixture for 30 minutes while stirring it magnetically to ensure an oxygen free atmosphere. Using an oil bath the flask was heated to 180 °C for 90 minutes. During the reaction a slight stream of nitrogen was maintained.
In both synthesis procedures a colour change of the suspension from bright red to deep black was observed that allowed to monitor the progress of the reaction. The work-up was identical for both methods: after cooling to room temperature the iron oxide coated alumina platelets (Fe3O4@Al2O3 platelets) were collected by vacuum filtration to separate them from the solvent and excessive, not adsorbed SPIONs. Afterwards they were washed with EtOH and dried in an evacuated desiccator over silica gel.
Polymer fibres were coated by first suspending 14.8 mg of aramid fibres or 82.7 mg of PVA fibres in 20 ml BnOH (99%) in 100 ml three neck round bottom flasks. After sonicating the suspensions for 5 minutes using a probe sonicator at 0.5 pulse and 40% intensity (UP200S, Dr Hielscher, Germany), fixed aliquots of 0.167 g of Fe(acac)3 (97%) in 2.5 ml BnOH or 0.2 g of Fe(acac)3 (97%) in 3 ml BnOH were added to the mixtures containing aramid or PVA fibres, respectively. The flasks were equipped with condensers connected to one-way gas releases and capped with rubber septae. The deoxygenation and heating procedure as well as the work-up was similar to the one described above for the Fe3O4@Al2O3 platelets.
For comparative purposes, iron oxide nanoparticles from a ferrofluid were also adsorbed on alumina platelets following the procedure described elsewhere in the literature.20 In brief, 0.5 g of alumina platelets were suspended in at least 10 ml of deionized water and appropriate amounts of a ferrofluid (EMG 705, Ferrotec, USA) diluted with deionized water were added to the suspensions so that a total volume of 11 ml was reached. The platelet suspensions were agitated for two days, filtered, washed with water and EtOH and vacuum dried.
Characterization of coated microparticles
The iron oxide coating on all microparticles was examined by scanning electron microscopy (SEM, LEO 1530, Zeiss, Germany). For Fe3O4@Al2O3 platelets, high-resolution transmission electron micrographs (HR-TEM) were acquired using a Philips Tecnai F30 TEM at 300 kV. The crystallographic nature of the iron oxide layer was determined by powder X-ray diffraction (XRD) on a PANalytical X'pert Pro diffractometer (Netherlands) equipped with a monochromator using Cu Kα-radiation. The specific surface area of as received and coated platelets was measured by BET nitrogen adsorption using a Nova 1000 (Quantachrome, USA).Magnetization curves were obtained in fields of up to ±1.5 T at 300 K using a Quantum Design (USA) Physical Property Measurement System (PPMS) with a 9 T superconducting magnet. To determine the magnetic susceptibilities of alumina platelets with varying amounts of iron oxide the dynamic magnetic properties of the samples were determined by measuring the amplitude dependence of the low-field AC susceptibility in the range 0.1–1.5 mT with a constant frequency of 1000 Hz.
Elemental analysis to determine the Fe concentration in iron oxide coated alumina platelets was performed as service by the Trace Element- and Microanalysis group of Prof. Günther at ETH Zürich (Switzerland) using inductively coupled plasma optical emission spectrometry (ICPOES, Arcos, Spectro, Germany). To prepare the measured solution ∼50 mg platelets were extracted in 2 ml 30% HCl assisted by a high pressure and temperature microwave and consequently diluted to match the calibration range. The following emission lines were monitored: Fe II 259.941, 238.204, 239.562 nm.
Alignment of magnetized microparticles
Fe3O4@Al2O3 platelets suspended in water were aligned using a rotating neodymium magnet (http://www.supermagnete.com, Switzerland). Iron oxide coated polymer fibres suspended in a viscous oil were aligned with static neodymium magnets. Light micrographs were obtained using a Leica DM IL LED inverted microscope equipped with a Leica DFC 295 camera (Leica, Germany).Synthesis of surface modifiers
The surface of Fe3O4@Al2O3 platelets was further functionalized with nitrodopamine (ND) derivatives to show the suitability of this approach to enable direct surface modification of magnetized microparticles. The detailed synthesis protocols to obtain the chemicals necessary for this surface modification are described in the ESI.†Adsorption of surface modifiers on magnetized platelets
The amount of nitrodopamine (ND) and nitrodopamine-palmitate (ND-PA) adsorbed on the surface of magnetized alumina platelets was determined by measuring the concentration of ND or ND-PA remaining in the supernatant solutions of settled suspensions using UV-Vis spectroscopy. ND is expected to show maximum adsorption close to the pKa of the molecule's –OH groups (pKa,1 = 6.2).33,34 However an orange solid precipitates when the pH of a ND-PA solution is increased towards the ideal value of 6.2. To avoid such precipitation, the adsorption isotherms for this molecule were obtained at the selected pH of 5.45. The detailed protocols used to prepare samples for adsorption measurements are described below.Preparation of platelet-stabilized Pickering emulsions
Pickering emulsions were prepared by manually shaking 1 ml water, 1 ml octane and 5 mg ND-PA coated Fe3O4@Al2O3 platelets in a 5 ml glass vial. Water in oil emulsions were obtained and collected at the bottom of the excess octane phase formed on the top of the vial. The emulsions were transferred onto a glass slide using a pipette for optical microscopy on a Leica DM IL LED inverted microscope equipped with a Leica DFC 295 camera (Leica, Germany).Results and discussion
Magnetic coating of Al2O3 microplatelets
Alumina microplatelets were successfully coated with a layer of magnetite nanoparticles (Fe3O4@Al2O3) via the non-aqueous sol–gel reaction of iron(III) acetylacetonate in benzyl alcohol at 180 °C using either microwaves or an oil bath as heat source (Fig. 1).35,36 Both protocols required an inert atmosphere to enable partial reduction of Fe(III) to Fe(II) and thus the formation of magnetite Fe3O4. Benzyl alcohol acts both as solvent and as reducing agent in this solvothermal sol–gel reaction.36 Due to the minimum quantities required for synthesis, the microwave route is convenient for screening experiments where a series of differently coated platelets are required. Since it allows batch sizes that are orders of magnitude larger than in the lab-scale microwave route, the oil bath method is particularly suited for experiments that demand larger amounts of identical platelets.In complementary experiments, we observed that the iron oxide coating can also be formed using melted polyethylene glycol (PEG) with molecular mass of 4000 g mol−1 as reaction medium.37 However, this route leads to slightly less homogeneous coatings, requires a higher reaction temperature of 270 °C, is a bit more laborious in the workup and thus was not further investigated in this study.
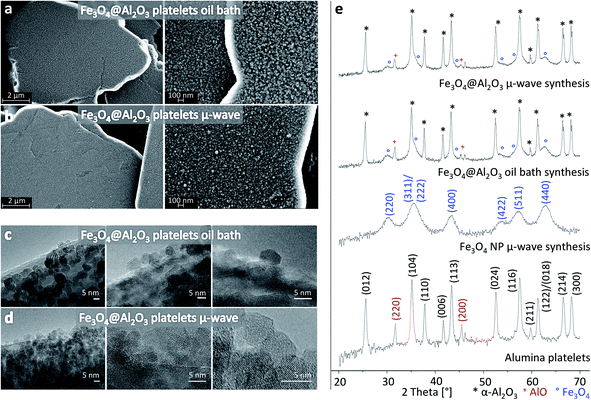
Electron microscopy and XRD measurements of the iron oxide coated alumina platelets show that the two protocols in benzyl alcohol lead to very similar homogeneous and dense coatings with Fe3O4 nanoparticles (Fig. 2). Such nanoparticle coating leads to nano-roughness on the surface of the platelets (Fig. 2a and b), which otherwise are predominantly smooth before the coating reaction (see ESI†). Transmission electron micrographs show that the size of particles within the coatings ranges from 4 to 9 nm (Fig. 2c and d). The coating is formed by a few layers of nanoparticles, leading to a typical total thickness of approximately 10 to 15 nm. The roughness and the possible presence of nanoporosity within the multi-layered coating increase the specific surface area of the microplatelets from 1.3 m2 g−1 to values within the range 7.4–9.3 m2 g−1 after the coating procedure.
The isotropic shape and faceted nature of the iron oxide nanoparticles suggest that they first nucleate homogeneously in solution and subsequently adsorb on the platelet surface. This is supported by the poor wetting of the nanoparticles on the platelet surface, as indicated by the dihedral angle higher than 90° of the iron oxide particles in direct contact with the alumina platelets (Fig. 2c and d). The differences in the crystal structures of the hexagonal corundum α-Al2O3 platelets and the cubic inverse spinel Fe3O4 nanoparticles likely prevents epitaxial growth of magnetite on the alumina platelet surface.
The formation of magnetic Fe3O4 as the main iron oxide phase within the coating is confirmed by powder X-ray diffraction measurements (Fig. 2e). The diffraction patterns of the as-received alumina platelets and of iron oxide nanoparticles synthesized using the microwave route in the absence of platelets were also recorded as references. The as-received alumina platelets consist mostly of α-Al2O3 with minor presence of AlO. Because of the small size of the nanoparticles the diffraction peaks of the iron oxide reference are very broad but can nevertheless be assigned to Fe3O4. This assignment is supported by Raman spectroscopy of Fe3O4@Al2O3 platelets (see ESI†). The diffraction patterns for the two samples of platelets coated by the different protocols are almost identical and can both be very well explained as a superposition of the two reference patterns. Thus, we conclude that the nanoparticle layers are composed of Fe3O4 independent of the heating method used.
Magnetic properties of Fe3O4@Al2O3 platelets
Fe3O4@Al2O3 platelets obtained from both synthesis routes show very similar superparamagnetic behaviour, as indicated by the cyclic magnetic measurements depicted in Fig. 3a. In both cases, a saturation magnetization of 2 A m2 kg−1 is obtained when normalized to the weight of the sample. The mass magnetic susceptibilities extracted from these curves are 0.12 m3 kg−1 and 0.10 m3 kg−1 for the iron oxide coated alumina platelets produced via the microwave and the oil-bath procedure, respectively.The magnetic properties of Fe3O4@Al2O3 platelets produced by the proposed non-aqueous sol–gel method were compared to those of platelets coated by the electrostatic adsorption of pre-formed iron oxide nanoparticles suspended in water (ferrofluid). For that purpose, we plotted the magnetic susceptibilities against the volume fraction of magnetite for samples prepared with different initial concentrations of iron oxide particles (ferrofluid route) or iron oxide precursors (sol–gel route) in Fig. 3b. We calculated the volume fraction of magnetite adsorbed on the platelet surface from the experimentally measured mass fraction of iron (for details see ESI†). Both types of coated platelets show a linear increase of the magnetic susceptibility with increasing iron oxide concentrations up to 0.9 vol% (1.2 wt% Fe). In this dilute regime, the magnetic susceptibility measured for the powder sample (χm,s) is expected to scale linearly with iron oxide concentration according to the relation: χm,s ∝ χm,npϕnp, where χm,np is the magnetic susceptibility of the nanoparticles and ϕnp is the volume fraction of nanoparticles with respect to the volume of the magnetized platelets. Thus, the ratio χferrom,s/χsol–gelm,s for different samples at one given volume fraction ϕnp should give a good approximation for the ratio χferrom,np/χsol–gelm,np for the different types of superparamagnetic nanoparticles. From the linear data shown in Fig. 3b (ϕnp < 0.9 vol% Fe3O4), we estimate that the magnetic susceptibility of the Fe3O4 nanoparticles prepared by the non-aqueous sol–gel route is a factor of three lower than that of the pre-formed iron oxide nanoparticles from the ferrofluid. This is likely a result of the different size and physical–chemical features of the ferrofluid particles and the nanoparticles made via sol–gel synthesis. The magnetic properties of superparamagnetic nanoparticles are known to depend strongly on several different parameters, such as structure, size, shape, anisotropy and surface chemistry.38–41
Using the ferrofluid route, the concentration of iron oxide nanoparticles could not be increased beyond the upper limit of 0.68 vol% Fe3O4 (0.64 wt% Fe). This is likely because of the electrostatic repulsion between the ferrofluid particles, which ultimately prevents the formation of a complete monolayer of nanoparticles on the platelet surface (see ESI†). On the contrary, the non-aqueous sol–gel synthesis allows us to reach significantly higher iron concentrations of up to 9.6 vol% Fe3O4 (8.8 wt% Fe). The Fe3O4@Al2O3 platelets magnetized by this procedure show lower magnetic susceptibility at the upper limit of the linear regime and a relatively constant susceptibility level for much higher iron oxide concentrations. A possible explanation for this behaviour beyond the linear range is that above a certain threshold concentration interactions between the individual magnetic dipoles of adjacent particles prevent them from freely orienting parallel to the direction of the external magnetic field.21 At high volume fractions of iron oxide these unfavourable interactions are more likely to occur since the particles and individual dipoles are closer to each other.
The iron oxide coating allows for control of the orientation and spatial distribution of magnetized platelets using low magnetic fields. For example, it was recently demonstrated that alumina microplatelets magnetized with iron oxide nanoparticles using the ferrofluid route can be effectively aligned in two dimensions by applying an external rotating magnetic field.20,21,25 Likewise, Fe3O4@Al2O3 platelets coated with magnetite by using the sol–gel approach show the same 2D alignment when subjected to rotating magnetic fields (Fig. 4). Such a response qualifies the homogeneously coated Fe3O4@Al2O3 platelets as suitable building blocks for the fabrication of composite materials with designed reinforcement architectures using low magnetic fields.20
Surface functionalization of magnetic Fe3O4@Al2O3 platelets
The magnetically responsive microplatelets prepared in this study can potentially be used as optical elements, sensing probes or reinforcing particles in composite materials. In many of these applications, surface functionalization of the microplatelets is crucial to enable for example mechanical coupling with the host material or anchoring of fluorescent molecules on the platelet surface. As opposed to the scattered sub-monolayer created by the ferrofluid route, the homogeneous iron oxide coating generated through the sol–gel method offers a convenient platform for the modification of the entire surface of the microplatelet with selected functional molecules.To demonstrate this feature, we studied the specific adsorption of molecular ligands that show high affinity for iron oxide surfaces. Catechols are well known for their ability to adsorb on a variety of different metal oxide materials,42 particularly on iron oxide surfaces, by coordinating to metal centres. Marine mussels exploit this ability in nature by using 3,4-dihydroxyphenylalanine rich polymers to strongly adhere their anchoring threads to the inorganic surface of rocks.43,44 In analogous synthetic systems, the catechol nitrodopamine was found to be an excellent ligand for the functionalization of Fe3O4 particles and surfaces.45–47
We take advantage of the high affinity of catechol towards iron oxide to functionalize the surface of the magnetically responsive Fe3O4@Al2O3 microplatelets. Indeed, nitrodopamine (ND) strongly adsorbs on the coated Fe3O4@Al2O3 platelets, reaching markedly higher adsorption densities than uncoated alumina particles, as shown in Fig. 5. The adsorption isotherm of ND on Fe3O4@Al2O3 platelets can be described using a two surface Langmuir model. In this model the amount of molecules adsorbed on the surface Cads is given by:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 l mmol−1, 2.34 μmol m−2, 246 l mmol−1 and 2.32 μmol m−2 for k1, Cmaxads,1, k2 and Cmaxads,2, respectively. This two-surface Langmuir model is often used to describe the adsorption of molecules on surfaces that change their electrical charges or degree of hydrophobicity depending on the actual concentration of adsorbed species. Changes in these important surface parameters eventually lead to two sets of equilibrium reactions between the ligand and the surface, which are characterized by two affinity constants (k1 and k2) and two maximum adsorption concentrations (Cmaxads,1 and Cmaxads,2). In our system, this behaviour might arise from the reversal of the electrical charge of the iron oxide surface upon increase of the concentration of ND. This is supported by the fact that ND (pKa,1 = 6.2)33 is partly deprotonated and thus negatively charged at the pH of the adsorption solution (pHads = 5.45), whereas Fe3O4 is positively charged at this pH (IEP = 6.7).46,50 Thus, the adsorption of ND on Fe3O4 is initially favoured by the electrostatic attraction between the oppositely charged adsorbate and surface. This is reflected in the high adsorption constant (k1) of 32
740 l mmol−1, 2.34 μmol m−2, 246 l mmol−1 and 2.32 μmol m−2 for k1, Cmaxads,1, k2 and Cmaxads,2, respectively. This two-surface Langmuir model is often used to describe the adsorption of molecules on surfaces that change their electrical charges or degree of hydrophobicity depending on the actual concentration of adsorbed species. Changes in these important surface parameters eventually lead to two sets of equilibrium reactions between the ligand and the surface, which are characterized by two affinity constants (k1 and k2) and two maximum adsorption concentrations (Cmaxads,1 and Cmaxads,2). In our system, this behaviour might arise from the reversal of the electrical charge of the iron oxide surface upon increase of the concentration of ND. This is supported by the fact that ND (pKa,1 = 6.2)33 is partly deprotonated and thus negatively charged at the pH of the adsorption solution (pHads = 5.45), whereas Fe3O4 is positively charged at this pH (IEP = 6.7).46,50 Thus, the adsorption of ND on Fe3O4 is initially favoured by the electrostatic attraction between the oppositely charged adsorbate and surface. This is reflected in the high adsorption constant (k1) of 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 l mol−1 obtained for low ND concentrations. Likely, the particle surface charge is shifted to lower values at higher ND contents, which decreases the electrostatic attraction between the adsorbate and the surface and results in a substantially lower binding constant (k2) of 246 l mol−1.50
740 l mol−1 obtained for low ND concentrations. Likely, the particle surface charge is shifted to lower values at higher ND contents, which decreases the electrostatic attraction between the adsorbate and the surface and results in a substantially lower binding constant (k2) of 246 l mol−1.50
 | ||
| Fig. 5 Adsorption isotherm of nitrodopamine on Fe3O4@Al2O3 platelets and on as-received alumina platelets. The sketch depicts schematically an adsorbed ND molecule on the iron oxide surface. | ||
Nitrodopamine (ND) is a versatile molecule that can be easily modified to serve as anchor group for the functionalization of iron oxide surfaces with a variety of different chemical groups. The primary amine can act as nucleophile in many different reactions. N-hydroxy succinimide (NHS) active esters are reagents that are preferentially attacked by amines rather than alcohols. Thus, the reaction of such active esters with ND offers an effective synthesis route to obtain a variety of ND derivatives.45,47,51 To illustrate the suitability of such chemistry for the surface modification of the magnetized microplatelets, we have exploited the NHS–ester amine coupling reaction to synthesize nitrodopamine palmitate (ND-PA) by reacting ND with N-hydroxy succinimide palmitate (NHS-PA).52,53 The reaction was conducted in DMF using NEt3 as base to abstract the proton from the amino group of ND (Fig. 6a). The product could be isolated as yellow powder by precipitation from EtOAc in a satisfying yield of 75.8%. 1H and 13C NMR spectroscopy as well as CHN analysis confirmed the successful synthesis of ND-PA. This is a new ND derivative that is expected to render Fe3O4 particles hydrophobic in a similar way as reported for nitroDOPA-based ligands with a hydrocarbon tail.12
To create hydrophobic magnetically responsive microparticles, ND-PA was adsorbed on Fe3O4@Al2O3 platelets for 24 h in an ethanolic solution with a concentration of 5.51 μmol ND-PA per gram solution. By comparing the dilution-corrected UV-Vis spectra of the ND-PA solution before and after the addition of platelets, we estimated the adsorption density of ND-PA on the alumina surface to be 1.5 μmol m−2 (Fig. 6b). Diffuse reflectance infrared Fourier transform (DRIFT)-spectra provide further evidence for the effective surface modification of the Fe3O4@Al2O3 platelets. Fig. 6c shows that the C–H stretches at 2921 cm−1 and 2850 cm−1 from ND-PA are readily visible in the spectrum of samples obtained after the adsorption. The signals from the aromatic C–C ring vibration (1494 cm−1) and the C–O stretch (1282 cm−1) of the adsorbed ND-PA are in very good agreement with literature values for adsorbed ND on iron oxide particles (1494 cm−1 and 1280 cm−1, respectively).46 The successful modification of the surface chemistry of the microparticles is promptly noticed in a very simple experiment: after adsorption of ND-PA on the surface of Fe3O4@Al2O3 platelets they become partially hydrophobic and can no longer be suspended in water (see images in ESI†).
As an exemplary possible application of such magnetically responsive anisotropic particles exhibiting surface hydrophobicity, Fe3O4@Al2O3 platelets modified with ND-PA are shown in Fig. 7 to effectively stabilize magnetically responsive water-in-oil emulsions by strongly adsorbing at the oil–water interface. The hydrophobized Fe3O4@Al2O3 platelets enable remote magnetic manipulation of the coated water droplets (see video in ESI†) and can potentially be more effective stabilizers of Pickering emulsions compared to spherical particles due to the stronger interfacial adsorption expected for anisotropic particle shapes.54
Magnetic coating of microfibers
The non-aqueous sol–gel procedure proposed here to coat alumina microplatelets with Fe3O4 can also be extended to microparticles with other geometries and chemistries. This versatility allows us to coat microparticles that cannot be easily magnetized via the adsorption of iron oxide nanoparticles from a ferrofluid. These include for example polymer microparticles that do not display electrical surface charges in aqueous media or microparticles that dissolve in water. To demonstrate the flexibility of the approach, we exploited the non-aqueous sol–gel method to modify polymer fibres of Aramid and poly(vinyl alcohol) (PVA). Fig. 8a, b, d and e shows that it is indeed possible to achieve a dense and homogeneous coating of iron oxide nanoparticles on the surface of these polymer microfibers. When subjected to a magnetic field the iron oxide coated polymer fibres showed a preferential direction of alignment parallel to the applied magnetic field (Fig. 8c and f). Since fibres are one-dimensional objects no rotating magnetic field is necessary in this case to achieve controlled alignment.20 When the direction of the magnetic field changes the fibre rotates and re-aligns its long axis parallel to the applied field due to the build up of a magnetic torque at the end of the particle (see video in ESI†). The non-aqueous coating procedure is also effective in depositing iron oxide nanoparticles on the surface of calcium sulphate rods, glass microfibers and mica flakes (results not shown).Conclusions
We have developed a non-aqueous sol–gel route to coat alumina microplatelets with a dense layer of superparamagnetic iron oxide nanoparticles. This iron oxide coating enables control of the spatial distribution and orientation of the Fe3O4@Al2O3 platelets using low magnetic fields. Besides magnetic response, the iron oxide coating also provides a convenient platform for further modification of the microparticle surface. As an example, we exploited the high affinity of nitrodopamine (ND) towards iron oxide to chemically modify the surface of Fe3O4@Al2O3 platelets with ND-PA, a hydrophobic ND-derived ligand. Fe3O4-coated platelets with such hydrophobic modification were used to create magnetically responsive Pickering emulsions. Finally, we showed that the proposed non-aqueous method can be easily adapted to other substrates, such as aramid or PVA microfibers. Such versatility makes this approach a convenient route for the preparation of magnetically responsive microparticles with multiple functionalities.Acknowledgements
We want to thank Antaria Ltd. for providing the alumina platelets, to the Polymer Technology group at ETH for providing the Twaron fibres, Prof. Ingo Burgert (ETH) for access to the Raman spectrometer and to ScopeM for access to TEM facilities. We acknowledge Dr Martin J. Süess for acquiring the TEM images, Doris Sutter for NMR spectroscopy, Michael Schneider for CHN-analysis and Kevin Guex for the quantitative iron analysis. Finally, we thank Dr Torben Gillich, Dr Davide Carnelli, Dr Jonathan Sander and Derya Erdem for helpful discussions on the organic synthesis, the iron oxide coating of polymer fibres, the formation of platelet stabilized emulsions and the adsorption of iron oxide particles on the platelets, respectively.References
- J. N. Anker, C. J. Behrend, H. M. Huang and R. Kopelman, J. Magn. Magn. Mater., 2005, 293, 655–662 CrossRef CAS PubMed.
- B. H. McNaughton, K. A. Kehbein, J. N. Anker and R. Kopelman, J. Phys. Chem. B, 2006, 110, 18958–18964 CrossRef CAS PubMed.
- M. M. Miller, P. E. Sheehan, R. L. Edelstein, C. R. Tamanaha, L. Zhong, S. Bounnak, L. J. Whitman and R. J. Colton, J. Magn. Magn. Mater., 2001, 225, 138–144 CrossRef CAS.
- A. Ghosh and P. Fischer, Nano Lett., 2009, 9, 2243–2245 CrossRef CAS PubMed.
- B. J. Nelson, I. K. Kaliakatsos and J. J. Abbott, Annu. Rev. Biomed. Eng., 2010, 12, 55–85 CrossRef CAS PubMed.
- C. Gosse and V. Croquette, Biophys. J., 2002, 82, 3314–3329 CrossRef CAS.
- J. S. Sander, R. M. Erb, C. Denier and A. R. Studart, Adv. Mater., 2012, 24, 2582–2587 CrossRef CAS PubMed.
- R. M. Erb, H. S. Son, B. Samanta, V. M. Rotello and B. B. Yellen, Nature, 2009, 457, 999–1002 CrossRef CAS PubMed.
- A. F. Ngomsik, A. Bee, M. Draye, G. Cote and V. Cabuil, C. R. Chim., 2005, 8, 963–970 CrossRef CAS PubMed.
- C. C. Berry and A. S. G. Curtis, J. Phys. D: Appl. Phys., 2003, 36, R198–R206 CrossRef CAS.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- E. Amstad, J. Kohlbrecher, E. Müller, T. Schweizer, M. Textor and E. Reimhult, Nano Lett., 2011, 11, 1664–1670 CrossRef CAS PubMed.
- I. S. Jacobs and C. P. Bean, Phys. Rev., 1955, 100, 1060–1067 CrossRef.
- P. G. de Gennes and P. A. Pincus, Phys. Kondens. Mater., 1970, 11, 189–198 CrossRef CAS.
- R. Blakemore, Science, 1975, 190, 377–379 CrossRef CAS.
- A. T. Skjeltorp, Phys. Rev. Lett., 1983, 51, 2306–2309 CrossRef CAS.
- D. van der Beek, A. V. Petukhov, P. Davidson, J. Ferre, J. P. Jamet, H. H. Wensink, G. J. Vroege, W. Bras and H. N. W. Lekkerkerker, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2006, 73, 041402 CrossRef CAS.
- M. A. Correa-Duarte, M. Grzelczak, V. Salgueiriño-Maceira, M. Giersig, L. M. Liz-Marzán, M. Farle, K. Sierazdki and R. Diaz, J. Phys. Chem. B, 2005, 109, 19060–19063 CrossRef CAS PubMed.
- J. Yuan, H. Gao, F. Schacher, Y. Xu, R. Richter, W. Tremel and A. H. E. Mueller, ACS Nano, 2009, 3, 1441–1450 CrossRef CAS PubMed.
- R. M. Erb, R. Libanori, N. Rothfuchs and A. R. Studart, Science, 2012, 335, 199–204 CrossRef CAS PubMed.
- R. M. Erb, J. Segmehl, M. Charilaou, J. F. Löffler and A. R. Studart, Soft Matter, 2012, 8, 7604–7609 RSC.
- R. M. Erb, J. Segmehl, M. Schaffner and A. R. Studart, Soft Matter, 2013, 9, 498–505 RSC.
- M. R. Sommer, R. M. Erb and A. R. Studart, ACS Appl. Mater. Interfaces, 2012, 4, 5086–5091 CAS.
- R. M. Erb, J. S. Sander, R. Grisch and A. R. Studart, Nat. Commun., 2013, 4, 1712 CrossRef PubMed.
- R. Libanori, R. M. Erb and A. R. Studart, ACS Appl. Mater. Interfaces, 2013, 5, 10794–10805 CAS.
- R. Libanori, F. B. Reusch, R. M. Erb and A. R. Studart, Langmuir, 2013, 29, 14674–14680 CrossRef CAS PubMed.
- R. T. Olsson, M. A. S. A. Samir, G. Salazar-Alvarez, L. Belova, V. Strom, L. A. Berglund, O. Ikkala, J. Nogues and U. W. Gedde, Nat. Nanotechnol., 2010, 5, 584–588 CrossRef CAS PubMed.
- E. Munch, M. E. Launey, D. H. Alsem, E. Saiz, A. P. Tomsia and R. O. Ritchie, Science, 2008, 322, 1516–1520 CrossRef CAS PubMed.
- C. Kuttner, A. Hanisch, H. Schmalz, M. Eder, H. Schlaad, I. Burgert and A. Fery, ACS Appl. Mater. Interfaces, 2013, 5, 2469–2478 CAS.
- T. Wang, A. B. Dalton and J. L. Keddie, Macromolecules, 2008, 41, 7656–7661 CrossRef CAS.
- L. M. Hamming, X. W. Fan, P. B. Messersmith and L. C. Brinson, Compos. Sci. Technol., 2008, 68, 2042–2048 CrossRef CAS PubMed.
- S. Lam, E. Blanco, S. K. Smoukov, K. P. Velikov and O. D. Velev, J. Am. Chem. Soc., 2011, 133, 13856–13859 CrossRef CAS PubMed.
- A. Palumbo, A. Napolitano, P. Barone and M. d'Ischia, Chem. Res. Toxicol., 1999, 12, 1213–1222 CrossRef CAS PubMed.
- P. C. Hidber, T. J. Graule and L. J. Gauckler, J. Eur. Ceram. Soc., 1997, 17, 239–249 CrossRef CAS.
- N. Pinna, S. Grancharov, P. Beato, P. Bonville, M. Antonietti and M. Niederberger, Chem. Mater., 2005, 17, 3044–3049 CrossRef CAS.
- I. Bilecka, I. Djerdj and M. Niederberger, Chem. Commun., 2008, 886–888 RSC.
- R. H. Goncalves, C. A. Cardoso and E. R. Leite, J. Mater. Chem., 2010, 20, 1167–1172 RSC.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. Vander Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS.
- C. Corot, P. Robert, J.-M. Idée and M. Port, Adv. Drug Delivery Rev., 2006, 58, 1471–1504 CrossRef CAS PubMed.
- V. Panchal, U. Bhandarkar, M. Neergat and K. G. Suresh, Appl. Phys. A: Mater. Sci. Process., 2014, 114, 537–544 CrossRef CAS.
- A. S. Teja and P.-Y. Koh, Prog. Cryst. Growth Charact. Mater., 2009, 55, 22–45 CrossRef CAS PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- J. H. Waite and M. L. Tanzer, Science, 1981, 212, 1038–1040 CAS.
- M. Yu, J. Hwang and T. J. Deming, J. Am. Chem. Soc., 1999, 121, 5825–5826 CrossRef CAS.
- E. Amstad, T. Gillich, I. Bilecka, M. Textor and E. Reimhult, Nano Lett., 2009, 9, 4042–4048 CrossRef CAS PubMed.
- E. Amstad, A. U. Gehring, H. Fischer, V. V. Nagaiyanallur, G. Hähner, M. Textor and E. Reimhult, J. Phys. Chem. C, 2011, 115, 683–691 CAS.
- T. Gillich, C. Acikgöz, L. Isa, A. D. Schlüter, N. D. Spencer and M. Textor, ACS Nano, 2012, 7, 316–329 CrossRef PubMed.
- G. Sposito, Soil Sci. Soc. Am. J., 1982, 46, 1147–1152 CrossRef CAS.
- A. R. Studart, E. Amstad and L. J. Gauckler, Langmuir, 2007, 23, 1081–1090 CrossRef CAS.
- E. Amstad, S. Zurcher, A. Mashaghi, J. Y. Wong, M. Textor and E. Reimhult, Small, 2009, 5, 1334–1342 CrossRef CAS PubMed.
- M. Rodenstein, S. Zürcher, S. G. P. Tosatti and N. D. Spencer, Langmuir, 2010, 26, 16211–16220 CrossRef CAS PubMed.
- Y. Lapidot, S. Rappoport and Y. Wolman, J. Lipid Res., 1967, 8, 142–145 CAS.
- J. Langecker, H. Ritter, A. Fichini, P. Rupper, M. Faller and B. Hanselmann, ACS Appl. Mater. Interfaces, 2012, 4, 619–627 CAS.
- B. P. Binks and T. S. Horozov, Colloidal Particles at Liquid Interfaces, Cambridge University Press, Cambridge, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed information (a) on the synthesis of ND and ND-PA, (b) on the Raman spectrum of Fe3O4@Al2O3 platelets, (c) on the determination of ND adsorption densities on Fe3O4@Al2O3 platelets including UV-Vis spectra, (d) on the measured Fe weight fractions and their conversion to Fe3O4 volume fractions, (e) on wettability experiments with different platelets and (f) on SEM analysis of as-received microparticles. We also show videos of the magnetic manipulation of iron oxide coated aramid fibres and of magnetically responsive Pickering emulsions. See DOI: 10.1039/c4ra09698c |
| This journal is © The Royal Society of Chemistry 2014 |