Synthesis of graphene-like g-C3N4/Fe3O4 nanocomposites with high photocatalytic activity and applications in drug delivery
Abstract
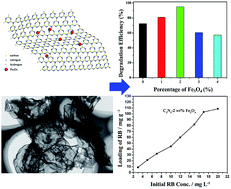
Graphene-like g-C3N4 nanosheet (GCN)/Fe3O4 quantum dot (QD) nanocomposites were successfully synthesized by a facile electrostatic self-assembly method. Characterization shows that the GCN is at least several micrometers in size. The GCN/Fe3O4 nanocomposites were used as photocatalysts for degradation of Rhodamine B (RhB) under visible light irradiation. After irradiation for 1.5 h, the degradation efficiency was 72.5% for pure g-C3N4, 81% for GCN-1 wt% Fe3O4, 95% for GCN-2 wt% Fe3O4, 60.46% for GCN-3 wt% Fe3O4 and 57.2% for GCN-4 wt% Fe3O4, indicating that GCN-2 wt% Fe3O4 nanocomposites had the highest photocatalytic activity. We deduce that the efficient separation of the photogenerated electron–hole pairs and the high specific surface area of GCN play important roles in the photocatalytic activity of the nanocomposites. In addition, the nanocomposites can be loaded with a model drug (Rhodamine B) and the loading capacity was as high as 108.6 mg g−1, making it a potential candidate for photocatalysis and controlled magnetically targeted drug delivery.

 Please wait while we load your content...
Please wait while we load your content...