Characterization and phenolation of biorefinery technical lignins for lignin–phenol–formaldehyde resin adhesive synthesis
Abstract
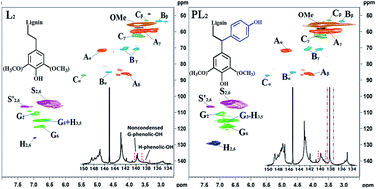
Technical lignins are cheap, abundant and renewable phenolic substances that have been attracting increasing attention. In this study, the structural features and active sites of four technical lignins obtained from different biorefinery processes were thoroughly characterized. Their suitability for partial incorporation into a phenol–formaldehyde (PF) resin adhesive was also evaluated. Phenolation treatment under alkaline conditions was conducted to enhance the reactivity of the technical lignins. Composition analysis indicated that all four technical lignins had a high purity (>88%). 13C nuclear magnetic resonance (NMR) and gel permeation chromatography (GPC) analyses revealed that the technical lignins from different original feedstocks and biorefinery processes had different structural features, but all of these technical lignins could be used in the synthesis of a lignin–phenol–formaldehyde (LPF) resin adhesive. The structural features and active sites of the different technical lignins before and after phenolation treatment were determined using quantitative two-dimensional heteronuclear single-quantum correlation (2D HSQC) and 31P NMR spectroscopies. The results confirmed that the phenolation treatment under alkaline conditions could effectively increase the number of active sites on the technical lignins and could be easily included in the synthesis process of LPF resin adhesives.

 Please wait while we load your content...
Please wait while we load your content...