BSA-conjugated CdS/Ag2S quantum dots: synthesis and preliminary antineoplastic assessment
Abstract
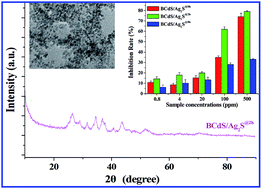
Tumours are one of the most difficult diseases to cure, and traditional chemotherapy drugs are always intimidating for use in tumour treatments because of their highly toxic side effects. This drives the research and development of innovative drugs. As a product, nanomaterials, particularly nano-drugs, are exhibiting great potential for use in tumour treatments. Based on the understanding of the Trojan horse-antineoplastic mechanism of nano-drugs, we tried to introduce the drug-combination opinion into nano-drugs and successfully developed a facile and eco-friendly synthesis route to fabricate bovine serum albumin-conjugated CdS/Ag2S quantum dots. It is notable that the double metallic sulphides showed a synergistic inhibition between Cd2+ and Ag+ on rat pheochromocytoma (PC 12) cells. Summarily, the present study substantiated the potential of synergistic anti-cancer opinion at the design of innovative nano-drugs with higher antineoplastic activity.

 Please wait while we load your content...
Please wait while we load your content...