Aqueous CO2 reduction on morphology controlled CuxO nanocatalysts at low overpotential
Abstract
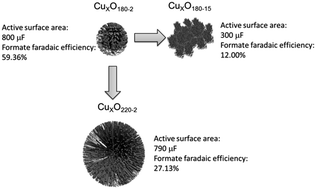
Various CuxO catalysts with different special microstructures were synthesized using a simple one-step hydrothermal method by controlling the reaction time and temperature conditions. Scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HR-TEM) were used to observe the morphologies of the received catalysts. The 3-dimensional (3D) hierarchical nanospheres (500 nm) comprised of secondary structured nanorods (50 nm) are formed at 180 °C for 2 hours. However, when increasing the hydrothermal reaction temperature to 220 °C, solid microspheres with a large size of 2.5 μm begin to appear instead of flabby hierarchical nanospheres. To further investigate the effect of morphologies on the activity and production selectivity of CuxO catalysts, cyclic voltammetry (CV) was used to evaluate the onset potential and current density of catalyzed CO2 reduction combining linear sweep voltammetry (LSV) in 0.5 M KHCO3 solution. The effect of catalyst loading was also tested by applying the gas diffusion layer (GDL) to make up a working electrode for CO2 electroreduction. The results indicate that the synthesized temperature of 180 °C for 2 h is the optimal condition for CuxO nanospheres and the optimal loading is about 3 mg cm−2, under which the onset potential for CO2 electroreduction reaches −0.55 V vs. SHE. By ion chromatography measurement, the faradaic efficiency and production rate of produced formate was found to be 59%, which is much higher than most reported Cu-based catalysts at the same electrolysis conditions, indicating the high selectivity of the CuxO nanospheres due to their controlled special surface morphology.
- This article is part of the themed collection: Materials for Energy storage

 Please wait while we load your content...
Please wait while we load your content...