Tuning nanoscale morphology using mixed solvents and solvent vapor treatment for high performance polymer solar cells†
Abstract
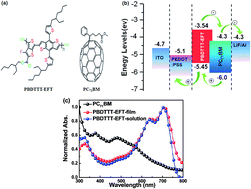
A series of high performance polymer solar cells (PSCs) were fabricated with poly[(4,8-bis-(2-ethylhexyloxy)-benzo[1,2-b:4,5-b′](dithiophene)-2,6-diyl-alt-(4-(2-ethylhexanoyl)-thieno [3,4-b]thiophene)-2,6-diyl] (PBDTTT-EFT) as the donor and with [6,6]phenyl-C71-butyric acid methyl ester (PC71BM) as the acceptor. The PSCs processed with DCB/CB mixed solvents show an open circuit voltage (Voc) of 0.79 V, a short circuit current density (Jsc) of 13.63 mA cm−2 and a fill factor (FF) of 62.9%, resulting in the highest PCE of 6.77% compared with a PCE of 5.99% for CB as a solvent and a PCE of 5.39% for DCB as a solvent. The PCE of PSCs processed with DCB/CB mixed solvents is further increased to 7.58% from 6.77% by chloroform vapor annealing treatment for 60 seconds. The PCE improvement should be attributed to the optimized bi-continuous interpenetrating networks of PBDTTT-EFT:PC71BM for better exciton dissociation and charge carrier collection.

 Please wait while we load your content...
Please wait while we load your content...