A FITC-doped silica coated gold nanocomposite for both in vivo X-ray CT and fluorescence dual modal imaging
Abstract
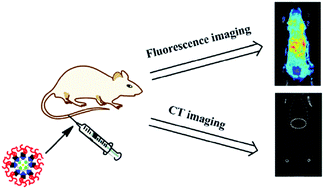
Dual-modal imaging contrast agents with unique X-ray computed tomography (CT) and optical imaging capabilities have attracted much attention in recent years. Herein, the new silica hybrid nanocomposites containing Au nanoparticles and FITC dyes (FITC–Au@SiO2) were investigated as this kind of contrast agent. TEM characterization showed that the as-synthesized FITC–Au@SiO2 nanoprobes were uniform in morphology with the average size of 90 nm. After the PEGylation, the obtained FITC–Au@SiO2@PEG exhibited good dispersion stability under different conditions. The MTT assay suggested that FITC–Au@SiO2@PEG didn't show appreciable toxicity toward KB cells even at high concentration of up to 1000 μg mL−1. In addition, it was found that FITC–Au@SiO2@PEG could cross the cell membrane and enter into the cytoplasm during the incubation with cells. In vivo dual modal optical/CT imaging of C57BL/6J mice uncovered that within 2 h post-injection FITC–Au@SiO2@PEG nanoprobes preferred to accumulate in the liver and were gradually cleared through the enterohepatic circulation system, which could bring novel opportunities to the next generation of dual-modality contrast agents.


 Please wait while we load your content...
Please wait while we load your content...