Synthesis of TiO2 hollow spheres using titanium tetraisopropoxide: fabrication of high efficiency dye sensitized solar cells with photoanodes of different nanocrystalline TiO2 sub-layers
Abstract
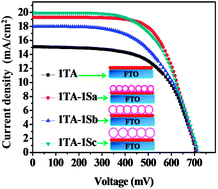
In this research TiO2 hollow spheres with different diameters were prepared using titanium tetraisopropoxide (TTIP) as the TiO2 precursor. Carbon spheres with average sizes of 230, 325 and 450 nm were prepared as the templates by hydrothermal method. Then TiO2 was deposited on the surface of the carbon spheres through a liquid phase deposition (LPD) process. This two dimensional growth was performed in an appropriate concentration of TTIP and different LPD times. Finally the TiO2 hollow spheres were achieved for specific LPD times by burning the carbon templates. Two kinds of TiO2 nanocrystals with sizes around 20 nm were hydrothermally grown in acidic (pH = 1.5) and basic (pH = 10) autoclaving pHs. These nanocrystals were deposited onto FTO glass substrates and applied in the photoanode of dye sensitized solar cells. The layers were transparent and demonstrated a strong bonding to the FTO substrates. Three kinds of TiO2 hollow spheres with external diameters around 310, 435 and 550 nm were also selected and used in a modified paste preparation process. They were finally deposited on the surface of transparent sub-layers of the TiO2 NCs to enhance the light scattering. According to the results, an energy conversion efficiency of 9.7% was achieved for the cell with 2TA-1Sc photoanode. This photoanode was composed of two sub-layers of TiO2 NCs prepared in acidic autoclaving pH and a layer of scattering hollow spheres. An efficiency of 9.4% was also attained for the cell with 1TB-1Sb photoanode including one layer of TiO2 NCs prepared in basic autoclaving pH and a layer of scattering hollow spheres. These efficiencies represented an increase of about 39% and 118% compared to those of the cells with 2TA and 1TB photoanodes, respectively.

 Please wait while we load your content...
Please wait while we load your content...