A phosphorus-, nitrogen- and carbon-containing polyelectrolyte complex: preparation, characterization and its flame retardant performance on polypropylene†
Abstract
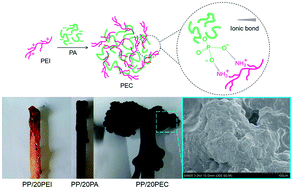
A new polyelectrolyte complex (PEC), containing phosphorus, nitrogen and carbon elements, has been prepared from positively charged polyethylenimine (PEI) and negatively charged phytic acid (PA). The PEC shows good performance in improving the thermo-oxidative stability and flame retardancy of pristine polypropylene (PP). When the content of PEC is 20 wt%, the char residues at 600 °C for the PP/PEC composites (PP/20PEC) are 10 wt% higher than those of pristine PP under an air atmosphere. Meanwhile, the peak heat release rate and total heat release of PP/20PEC are 314 W g−1 and 7.7 kJ g−1 lower than those of pristine PP, respectively. In comparison, PEI or PA is less effective than PEC in improving the thermo-oxidative stability and flame retardancy of pristine PP. Furthermore, the char residues of PP/20PEC are more full and compact, the surfaces of which are covered with a perfect intumescent layer. This work proposes a new guideline for designing and fabricating an intumescent flame retardant system.

 Please wait while we load your content...
Please wait while we load your content...