Simulation of an axial–radial ammonia synthesis reactor by linking COMSOL–MATLAB software
Abstract
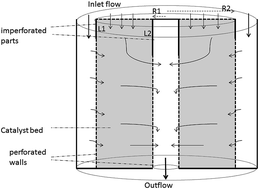
In this work a two dimensional mathematical model is developed for simulation of axial–radial ammonia synthesis reactors. Mass and energy balance equations are solved by a finite volume method using MATLAB (2012b) software. Momentum and general continuity equations, considering the effect of solid particles, are handled by COMSOL Multiphysics (4.3a). COMSOL is a Finite Element Method (FEM) software package for solving partial differential equations. By linking COMSOL and MATLAB software, momentum, mass, and energy balance equations are solved simultaneously. The MATLAB–COMSOL-LiveLink combines the powerful FEM abilities of COMSOL with the versatile programming environment of MATLAB. The effectiveness factor is calculated by the boundary value differential equation to consider the diffusion effect in catalyst particles. Furthermore the effect of pressure on ammonia concentration in the reactor is studied. Finally, simulation results are validated by the practical data of the Shiraz petrochemical plant.

 Please wait while we load your content...
Please wait while we load your content...